Poster Session
Myositis
Poster Session A
Session: (0280–0305) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster I
0296: Aggregation of HARS1 and Internalized Antibodies in Muscle Biopsies of Patients with Antisynthetase Syndrome and anti-jo1(hars) Autoantibodies
Sunday, October 26, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
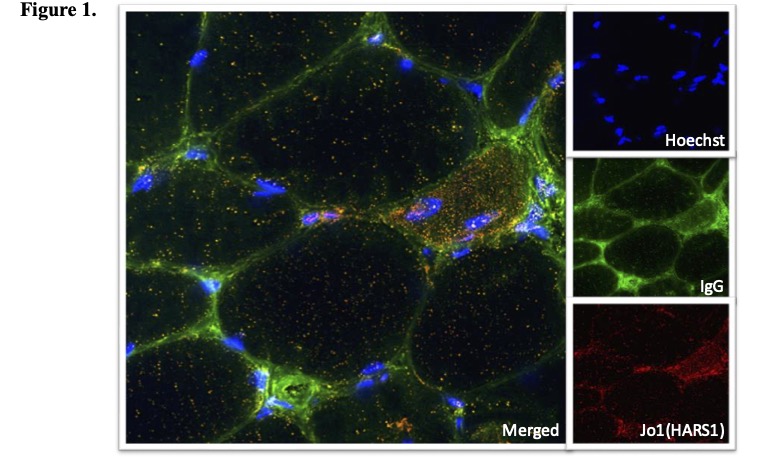
Direct immunofluorescence showing the aggregation of HARS1 and internalized antibodies in a muscle biopsy of a patient with antisynthetase syndrome and anti-Jo1 (HARS1) autoantibodies
- MC
Maria Casal-Dominguez, Staff Scientist
National Institutes of Health
Bethesda, Maryland, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: Autoimmune inflammatory myopathies (IMs) comprise a diverse group of diseases that primarily affect the muscles and often involve the lungs, skin, and joints. Among them, patients with autoantibodies against aminoacyl-tRNA synthetases develop a distinct clinical entity known as antisynthetase syndrome (ASyS), characterized by inflammatory myopathy, pulmonary fibrosis, arthritis, Raynaud’s phenomenon, and mechanic’s hands. The most common antisynthetase autoantibody, anti-Jo1, targets histidyl-tRNA synthetase (HARS).
Recent studies have demonstrated that autoantibodies can penetrate muscle fibers and disrupt the function of their target autoantigens. In ASyS patients, this process induces a distinct transcriptomic profile that recapitulates the effects of pharmacologic inhibition of HARS with histidinol.
This study aims to examine the localization of anti-Jo1 antibodies and their cognate antigen, HARS1, in muscle biopsies from anti-Jo1–positive ASyS patients and diseased controls.
Methods: We performed direct immunofluorescence for IgG and HARS1 staining on 10 μm-thick unfixed muscle sections from patients with anti-Jo1 autoantibodies and those with other forms of inflammatory myopathy. Images were acquired using a Leica SP8 high-resolution confocal microscope.
Results: We observed co-aggregation of HARS1 and internalized IgG in muscle biopsies from ASyS patients with anti-Jo1 autoantibodies (Figure 1). This pattern was not detected in samples from patients with other forms of myopathy.
Conclusion: Our data suggest that antibodies from anti-Jo1 patients induce HARS1 aggregation in the cytoplasm of muscle fibers. Notably, similar HARS1 aggregation has been observed in patients with HARS1 mutations and Charcot-Marie-Tooth (CMT) disease. In CMT, this aggregation disrupts HARS1 function, resulting in errors in peptide translation.

