Poster Session
Rheumatoid Arthritis (RA)
Poster Session A
Session: (0470–0505) Rheumatoid Arthritis – Treatment Poster I
0471: An Open-label, Randomized, Controlled Phase 1/2 Study to Assess the Safety and Efficacy of KYV-101 anti-cd19 CAR-T Cell Therapy in Active and Difficult-to-treat ACPA Positive Rheumatoid Arthritis: Preliminary Results of the COMPARE Trial
Sunday, October 26, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
- IM
Ioanna Minopoulou, MD;MSc
Department of Rheumatology and Clinical Immunology, Charité - Universitätsmedizin Berlin, Berlin, Germany
Berlin, GermanyDisclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: Rheumatoid Arthritis (RA) is a chronic autoimmune disease characterized by autoreactive B cells that produce anti-citrullinated protein antibodies (ACPA), contributing to sustained synovial inflammation and joint destruction. Recent clinical reports indicate that anti-CD19 chimeric antigen receptor (CAR) T-cell therapy shows promise in inducing drug-free clinical remission in difficult-to-treat RA (D2T-RA, Lidar 2025; Li 2025). The COMPARE (COMparison of B-cell dePletion by rituximAb and anti-CD 19 CAR-T therapy in Patients with rhEumatoid arthritis) trial was designed to (i) evaluate the safety and efficacy of the anti-CD19 CAR-T cell therapy KYV-101 in ACPA-positive patients with active, treatment refractory D2T-RA and (ii) to compare the impact of KYV-101 on disease activity to the standard-of-care anti-CD20 monoclonal antibody rituximab (RTX). KYV-101 is a fully human, autologous anti-CD19 CAR T-cell therapy with CD28 costimulation, which is under investigation in B-cell driven autoimmune disease. With a single administration, KYV-101 has potential to achieve durable drug-free and disease-free remission via deep B-cell depletion and immune reset. Preliminary data suggest its potential for durable effects across rheumatic (Albach 2025, Minopoulou 2025, Haase 2025) and neuroimmune indications (Haghikia 2023; Faissner 2024).
Methods: COMPARE, an open-label, randomized, controlled Phase 1/2 trial (EU CT:2024-514955-13-00) is currently enrolling the safety run-in of the trial (Phase 1; n=6) during which all participants receive 1×108 CAR T cells after lymphodepletion. The primary endpoint in Phase 1 is safety (Cytokine Release Syndrome (CRS), Immune Cell Associated Neurotoxicity Syndrome (ICANS), adverse events). Additional endpoints include pharmacokinetics of KYV-101, B-cell and autoantibody levels, and disease activity scores.
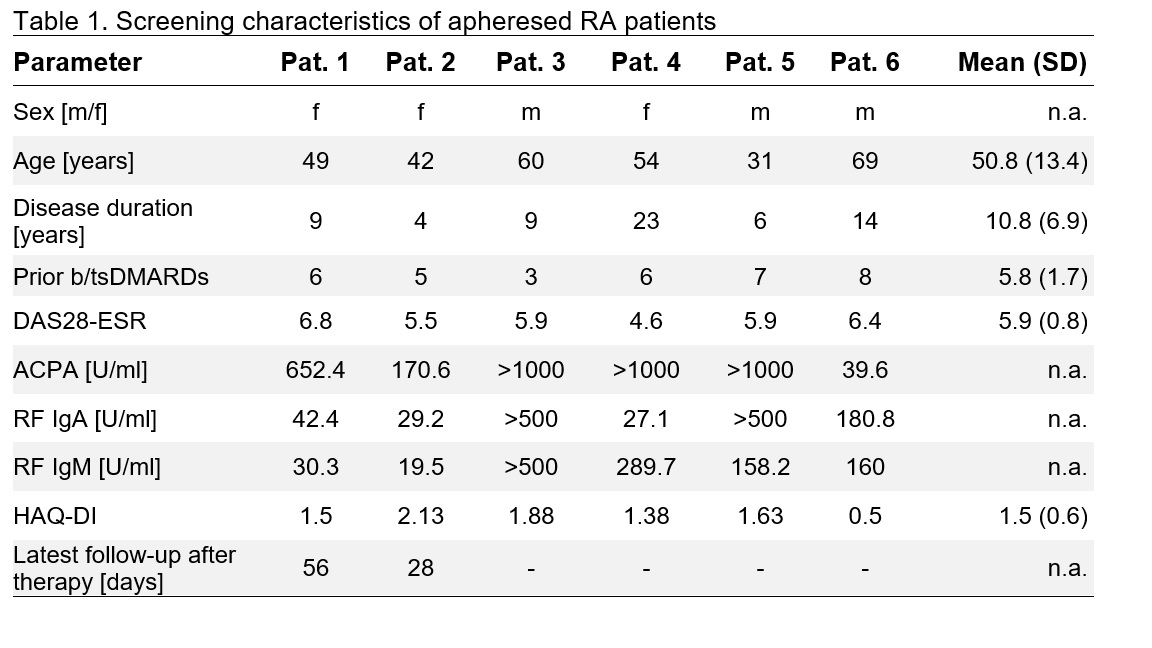
Results: At the time of submission, Phase 1 is fully recruited with 6 participants apheresed; two participants have received KYV-101 by 12 May 2025, with follow-ups up to 28 and 56 days. Participants had active disease for a mean of 10.8 (±6.9) years duration and 5.8 (±1.7) prior therapies (Table 1). Both treated participants experienced expected transient cytopenias due to lymphodepletion and CRS (grade I and II), which promptly resolved following tocilizumab and dexamethasone administration. No ICANS, serious adverse events or deaths occurred. CAR T-cells expanded rapidly, reaching peaks of 479 and 122 cells/mL at days 21 and 10 after KYV-101 infusion. Both patients exhibited complete peripheral B-cell depletion and a decline in ACPA values to 19% and 5% of screening values at the latest follow-up.
Conclusion: Preliminary data from the COMPARE trial suggest that KYV-101 has a favorable safety profile and leads to robust CAR T-cell expansion and complete peripheral B-cell depletion in seropositive, active, treatment refractory D2T-RA. With apheresis completed and planned KYV-101 infusion for all Phase 1 patients by July 2025 completion of safety run-in is anticipated shortly, followed by the randomized portion of the trial. Updated data summarizing Phase 1 experience with estimated follow-up post-infusion of 4 months will be presented.

