Poster Session
Epidemiology, Health Policy, and Outcomes
Poster Session A
Session: (0210–0232) Measures & Measurement of Healthcare Quality Poster I
0232: Hydroxychloroquine Blood Testing in Lupus: The Michigan Medicine Experience
Sunday, October 26, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
.jpg)
Michigan Medicine HCQ Blood Level Target Range Guidelines
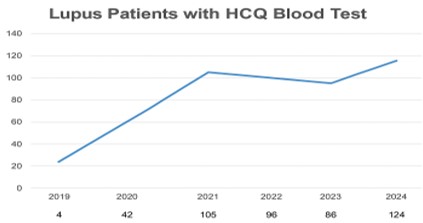
Number of Michigan Medicine lupus patients with HCQ blood tested between 2019 and 2024
.jpg)
HCQ Blood Level Distribution in Our Patient Cohort
- ZS
Zeinab Saleh, MD
University of Michigan
Ann Arbor, MI, United StatesDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Background/Purpose: Measuring hydroxychloroquine (HCQ) blood levels in patients with systemic lupus erythematosus (SLE) can assess medication adherence and determine whether concentrations fall within the therapeutic or toxic ranges. Despite this potential, HCQ monitoring has not been incorporated into routine SLE care, largely due to the absence of standardized clinical cut-offs and formal guidelines from rheumatology associations (e.g, the American College of Rheumatology and the European Alliance of Associations for Rheumatology). Our objectives are twofold: (1) to provide evidence-informed recommendations for monitoring HCQ blood levels in adult SLE patients on therapy for at least six months, and (2) to evaluate HCQ levels in our lupus population at Michigan Medicine (MM).
Methods: We conducted a systematic review using PubMed, Embase, and Cochrane databases (from inception to October 2024), applying MeSH terms and keywords related to lupus and hydroxychloroquine. A multidisciplinary team—including representatives from rheumatology, dermatology, ophthalmology, nursing, pharmacy, pathology, the Internal Medicine Clinical Experience & Quality team, and a patient advisor—held six teleconferences, reached consensus, and developed institutional recommendations for HCQ blood level monitoring. In parallel, our continuous improvement specialist and data analyst assisted in extracting data from our adult lupus patient population.
Results: (1) Our team defined target HCQ blood level ranges for four clinical categories: severe non-adherence (frequent interruptions or complete discontinuation), partial non-adherence (occasional missed doses) or subtherapeutic levels, therapeutic levels, and supra-therapeutic levels (associated with potential retinal toxicity). These guidelines are now posted on PolicyStat, Michigan Medicine’s centralized platform for institutional policies, procedures, and guidelines (Figure 1).
(2) From January to July 2024, only 4% of adult lupus patients managed by MM Rheumatology had their HCQ blood levels tested. However, utilization has steadily increased since the test was introduced in 2019 (Figure 2). Figure 3 illustrates the distribution of HCQ levels among our tested patients.
Conclusion: Our work provides clinicians with practical, consensus-based guidance for monitoring HCQ blood levels in patients with SLE. We are actively engaging stakeholders- including rheumatologists, ophthalmologists, dermatologists, nurses, and patients- to raise awareness of the benefits of HCQ level monitoring in improving long-term outcomes. Our goal is to increase testing among HCQ-treated lupus patients from 4% to 25% within the next six months.

