Poster Session
Antiphospholipid Syndrome
Poster Session A
Session: (0115–0144) Antiphospholipid Syndrome Poster
0143: High-dimensional Spectral Flow Cytometry Reveals a Unique Distribution of Circulating B Cells in Patients with Antiphospholipid Syndrome
Sunday, October 26, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
.jpg)
Figure 1. Gating strategies of peripheral B cells from PBMCs.
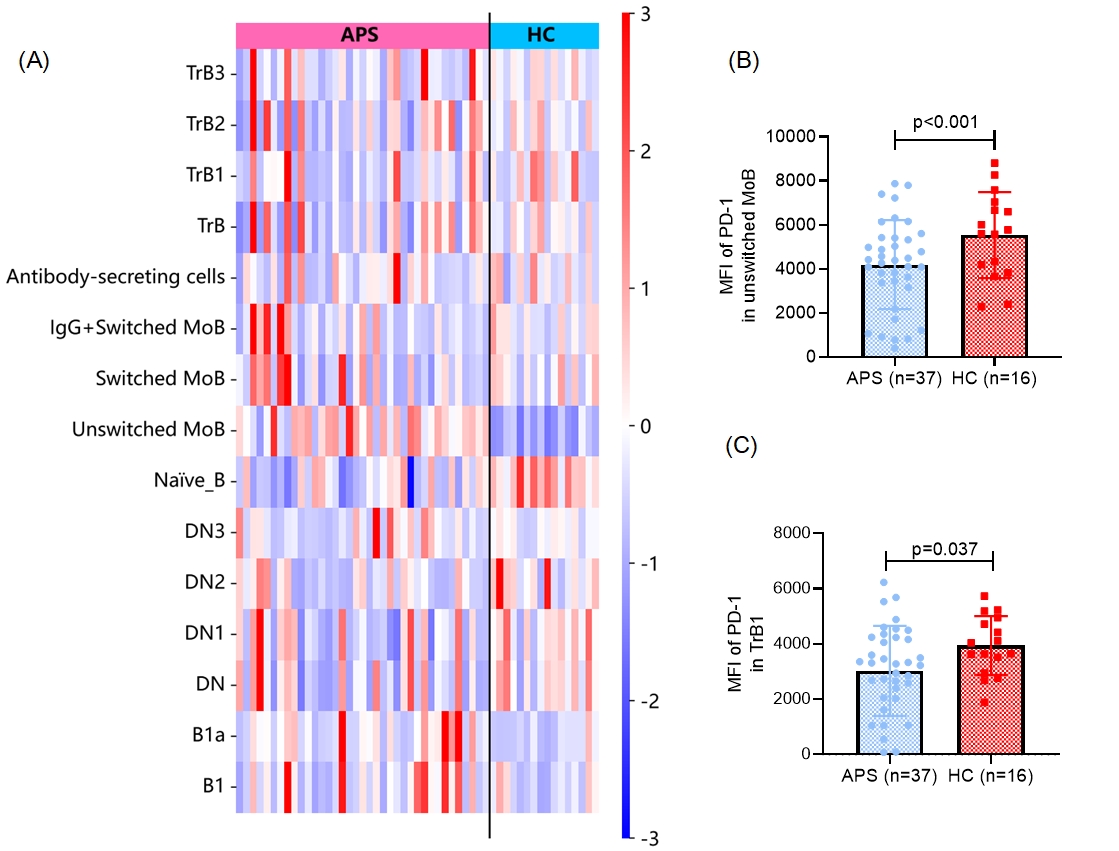
Figure 2. Altered proportions of B cell subsets and reduced PD-1 expression on unswitched memory and transitional B1 cells in APS patients.
(A) Heatmap showing the relative percentages (Z-score normalized) of B cell subsets in peripheral blood from patients with antiphospholipid syndrome (APS, n = 37) and healthy controls (HC, n = 16). Subsets include transitional B cells (TrB, TrB1–TrB3), memory B cells (MoB, switched and unswitched), antibody-secreting cells, naïve B cells, double-negative B cells (DN, DN1-DN3), and B1 cells (B1, B1a).
(B) Median fluorescence intensity (MFI) of PD-1 on unswitched memory B cells is significantly lower for APS patients versus HCs.
(C) PD-1 MFI on transitional B1 cells is also reduced in APS patients.
(A) Heatmap showing the relative percentages (Z-score normalized) of B cell subsets in peripheral blood from patients with antiphospholipid syndrome (APS, n = 37) and healthy controls (HC, n = 16). Subsets include transitional B cells (TrB, TrB1–TrB3), memory B cells (MoB, switched and unswitched), antibody-secreting cells, naïve B cells, double-negative B cells (DN, DN1-DN3), and B1 cells (B1, B1a).
(B) Median fluorescence intensity (MFI) of PD-1 on unswitched memory B cells is significantly lower for APS patients versus HCs.
(C) PD-1 MFI on transitional B1 cells is also reduced in APS patients.
.jpg)
Table 1. Baseline Demographic Characteristics of APS Patients and Healthy Controls
- YG
Yuzhou Gan, MBBS
University of Michigan
Ann Arbor, Michigan, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: Background: Antiphospholipid syndrome (APS) is a systemic autoimmune disorder characterized by the presence of pathogenic antiphospholipid antibodies (aPL) in the setting of venous or arterial thrombosis, pregnancy morbidity, and other morbid manifestations. B cells are central to APS pathogenesis through their role in aPL production. However, despite their pathogenic importance, the phenotypic and functional characteristics of circulating B cells in APS remain incompletely understood. In this study, we applied high-dimensional spectral flow cytometry to comprehensively characterize the activation profiles of circulating B cells in patients with APS.
Methods:
Methods: We developed a 26-color spectral flow cytometry panel to deeply characterize circulating B cell subsets and their activation states in patients with APS. The panel was designed based on established literature and included markers for B cell development, activation, memory, homing, and regulatory phenotypes. Peripheral blood mononuclear cells (PBMCs) were isolated from APS patients (n=37) fulfilling the 2023 ACR/EULAR APS classification criteria and age- and sex-matched healthy donors (n=16). Samples were stained and analyzed using a 5-laser Cytek Aurora spectral flow cytometer. Manual gating was performed using FlowJo software to define major B cell subsets, including naïve, transitional, memory, double-negative, and plasmablast populations. Activation markers such as CD86, CD95, CD69, and HLA-DR were assessed across subsets. Statistical analyses were conducted using SPSS to compare APS patients with healthy controls, with significance defined as p < 0.05.
Results: Results: Among the patients, 25 (67.6%) were diagnosed with primary APS, while 12 (32.4%) had APS associated with systemic lupus erythematosus (SLE). Gating strategies were shown in Fig. 1. Compared with healthy controls, patients with APS exhibited an increased proportion of B1a cells (CD5+CD43+) (0.30% of CD19+CD20+B cells [0.15, 0.44] vs. 0.14 [0.08, 0.22], p=0.010) and unswitched memory B cells (CD27+IgD+IgM+) (30.52% of CD19+CD20+B cells [21.68, 36.79] vs. 12.19 [9.59, 17.58], p< 0.001). In contrast, the proportion of naïve B cells (CD27-IgD+IgM+) was significantly reduced in APS patients (46.21% of B cells [42.34, 58.44] vs. 63.01 [57.51, 72.64], p< 0.001) (Fig. 2A). Moreover, the expression of the key checkpoint regulator PD-1 was reduced on transitional B1 cells (CD10hiIgDhiCD24hiCD38hi) and unswitched memory B cells in APS patients (Fig. 2B-C).
Conclusion: High-dimensional profiling of circulating B cells in APS patients reveals distinct alterations in subset distribution and activation states, including increased B1a and unswitched memory B cells and reduced naïve B cells, alongside diminished PD-1 expression on key populations. These results provide novel insights into B cell dysregulation in APS and raise the possibility that augmenting PD-1 signaling or modulating specific B cell subsets could represent future therapeutic strategies for this complex autoimmune disorder.

