Poster Session
Vasculitis
Poster Session B
Session: (1612–1632) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
1613: Switching From Reference Tocilizumab to Biosimilar in Giant Cell Arteritis: Effectiveness and Safety in a Multicenter Study of 38 Patients
Monday, October 27, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
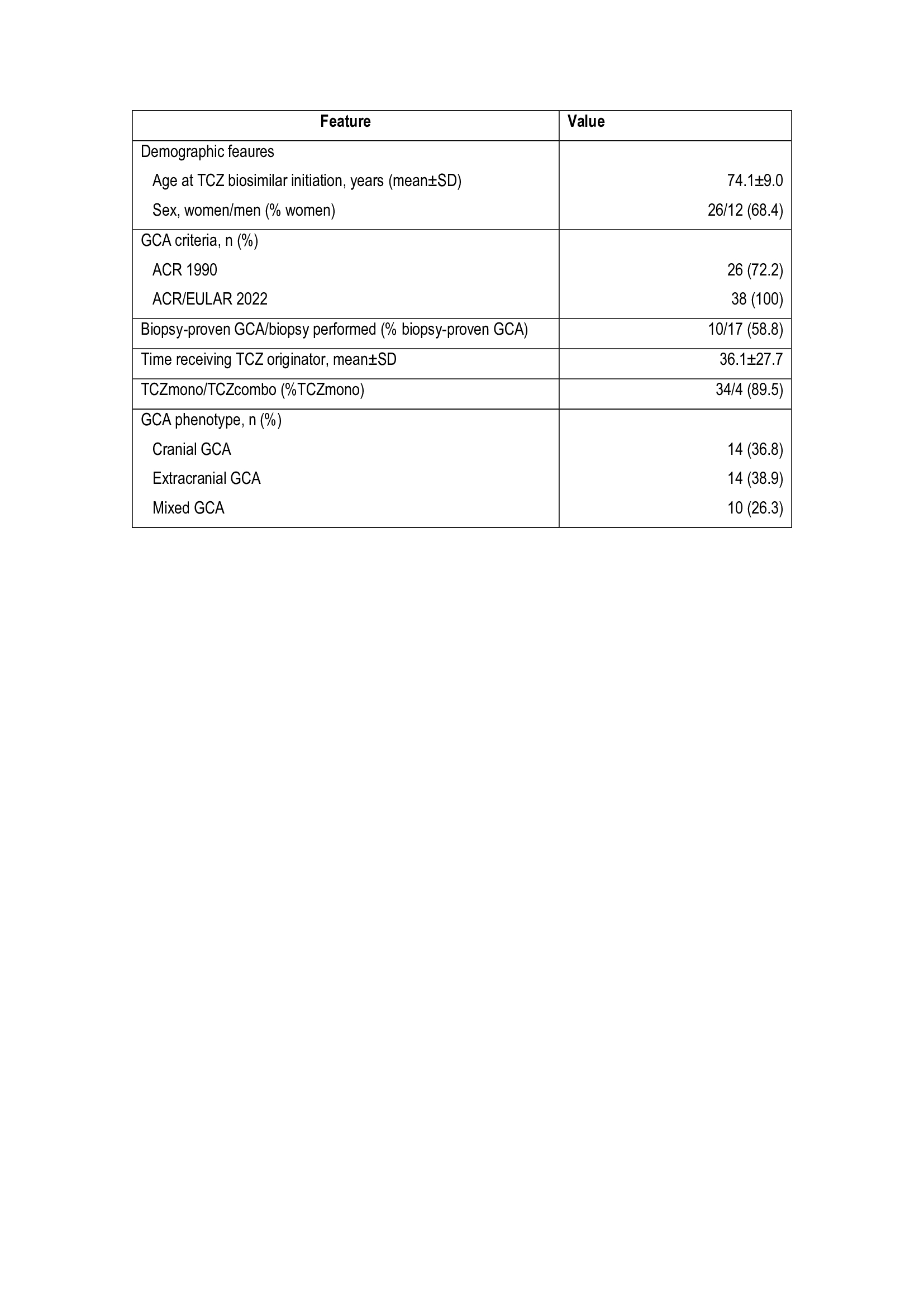
TABLE. Main features of the 30 GCA patients who were switched from TCZ - reference product to TCZ- biosimiliar.
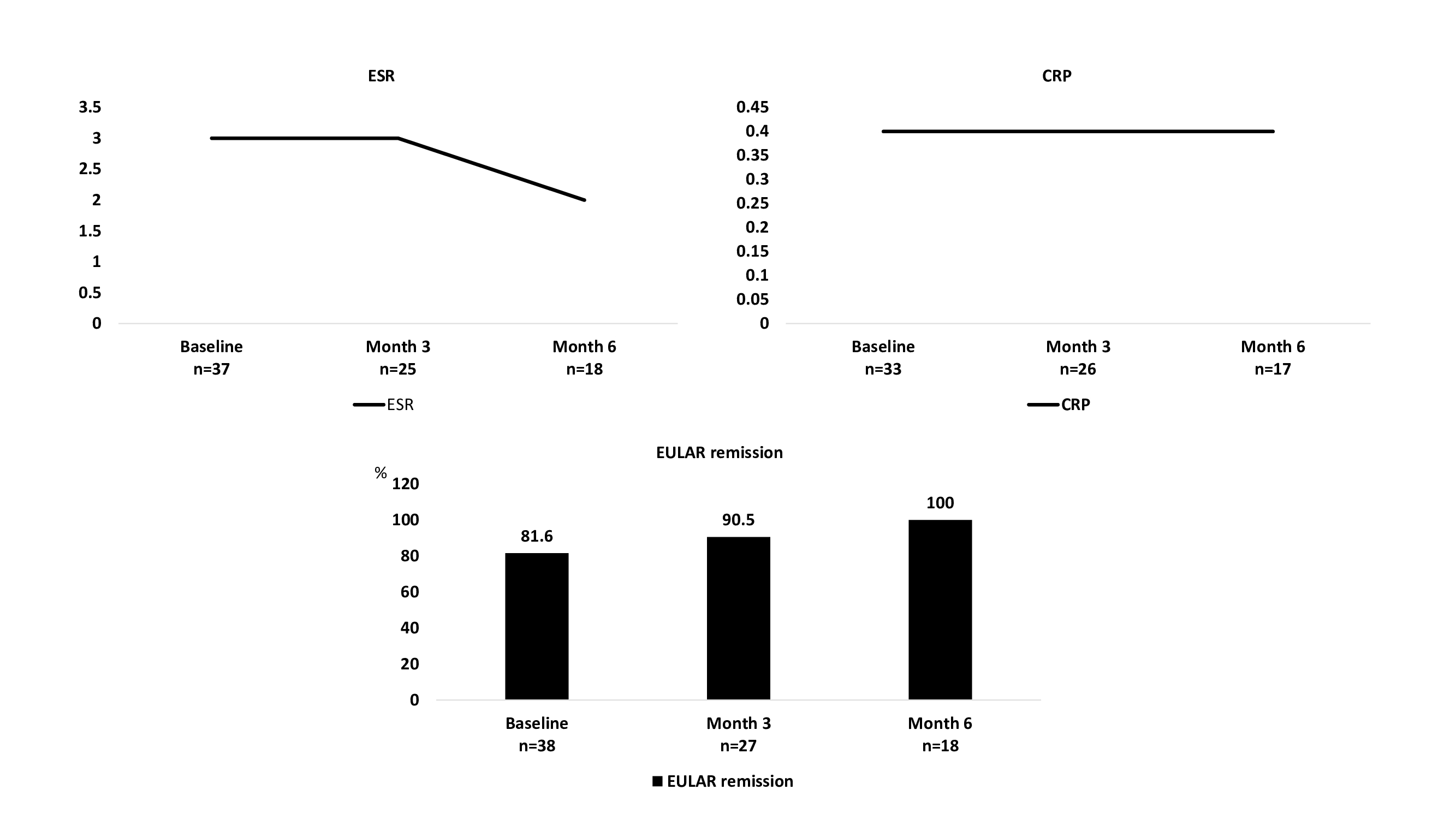
FIGURE. Median ESR, CRP and daily prednisone dose, and EULAR remission of the 38 GCA patients who were switched from TCZ - reference product to TCZ- biosimiliar.
- AM
Adrián Martín-Gutiérrez, -None-
Hospital Universitario Marques de Valdecilla, IDIVAL
Renedo de Piélagos, SpainDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Background/Purpose: The cost of original biologics may limit treatment access. Biosimilars are biologics that are structurally highly similar and functionally equivalent to the approved reference product, providing the opportunity to improve access to treatment. Tocilizumab (TCZ)-biosimilar have showed therapeutic equivalence in rheumatoid arthritis. To date, there are no studies evaluating the effectiveness and safety of the switch to TCZ-biosimilar in GCA. The aim of this study is to assess the effectiveness and safety of switching from TCZ-reference product to TCZ-biosimilar in GCA patients in clinical practice.
Methods: Retrospective, multicentre, real-world clinical practice study of GCA patients who switched from TCZ-reference product to TCZ-biosimilar. We assessed a) effectiveness including clinical and laboratory parameters, and EULAR definition of remission (absence of signs and symptoms and normalization of acute phase reactants) (5), b) dose of prednisone, and c) side effects. Comparisons were performed between baseline and 3rd, and 6th months using McNeemar´s test for binary variables and T-student test for continuous variables
Results: A total of 38 patients (68.4% females, mean age 74.1 years) who were switched from TCZ-reference product to TCZ-biosimilar were included for analysis (TABLE). The GCA phenotypes were: cranial (n=14; 36.8%), extracranial (n=14; 38.9%) and mixed (n=10; 26.3%). The patients had received TCZ-reference product for 36.1±27.7 months. Tyenne was prescribed in monotherapy in 34 (89.5%) patients, and combined with methotrexate in 4 (10.5%) patients. At the time of switching from TCZ-reference product to TCZ-biosimilar, 31 patients were in EULAR remission, the median (IQR) prednisone dose was 0 [0-5] mg/day, and the median ESR and CRP were 3 [2-8] mm/1st hour, and 0.4 [0.1-0.4] mg/dL, respectively. After a mean follow-up of 7.5±3.4 months from switching to TCZ biosimilar, there were no significant differences in EULAR remission, daily prednisone dose and CRP and ESR values (Figure). Due to GCA remission TCZ administration interval was increased in 4 (10.5%) patients and discontinue in other. One patient developed herpes zoster one month after switching fromTCZ-reference product to TCZ-biosimilar
Conclusion: Our results suggest that TCZ-biosimilar is an effective and safe treatment for GCA patients when they were switched from the TCZ-reference product.
References
1. Calderón-Goercke M, et al. Clin Exp Rheumatol. 2020. PMID: 32441643
2. Loricera J et al. Clin Exp Rheumatol. 2014. PMID: 24854377
3. Prieto Peña D, et al. Clin Exp Rheumatol. 2021. PMID: 33253103
4. Loricera J, et al. Ther Adv Musculoskelet Dis. 2022. PMID: 35898567
5. Dejaco C, Kerschbaumer A, Aletaha D, Bond M, Hysa E, Camellino D, et al. Treat-to-target recommendations in giant cell arteritis and polymyalgia rheumatica. Ann Rheum Dis 2024. PMID: 36828585

