Poster Session
Systemic Lupus Erythematosus (SLE)
Poster Session A
Session: (0641–0670) Systemic Lupus Erythematosus – Treatment Poster I
0656: Low-dose belimumab reduced disease flares in patients with systemic lupus erythematosus at low disease activity: a multicenter, randomized, double-blind, placebo-controlled trial
Sunday, October 26, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
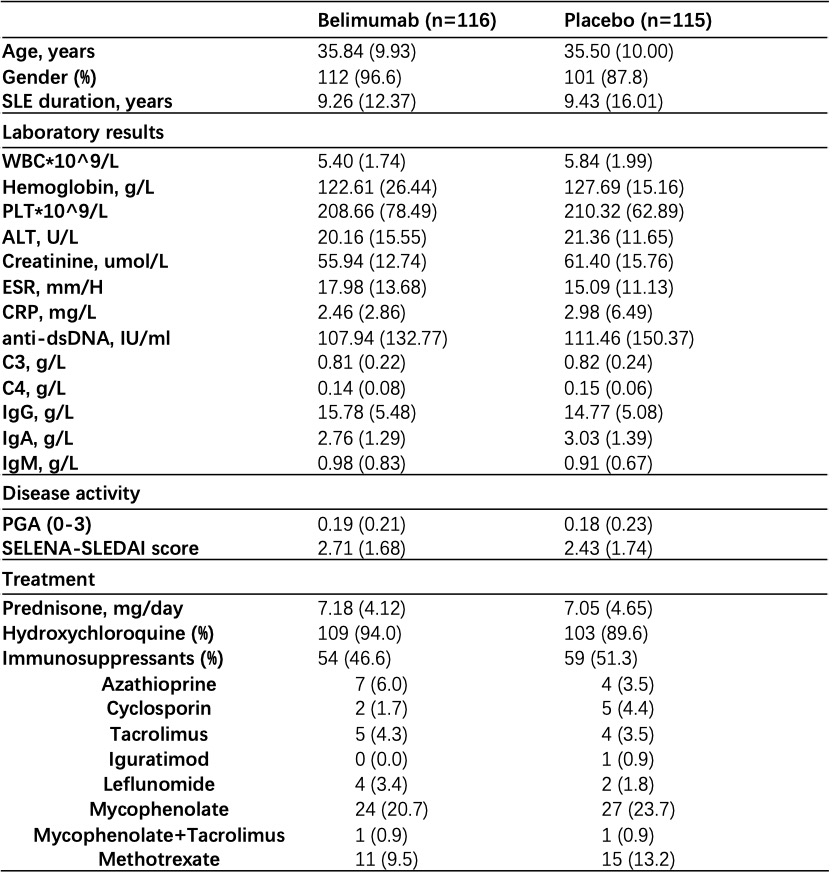
Table 1. Baseline characteristics of participants in two arms.
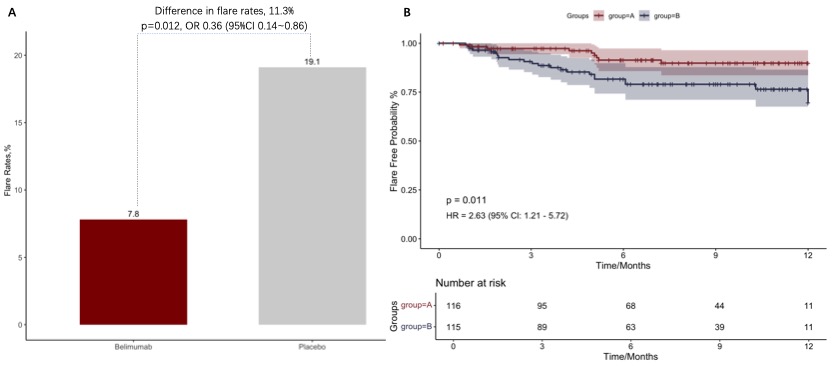
Figure 1. The difference of overall disease flares between low-dose belimumab and placebo on the basis of SOC (A). Kaplan-Meier Curves demonstrated flare-free survival of participants from two arms (B).
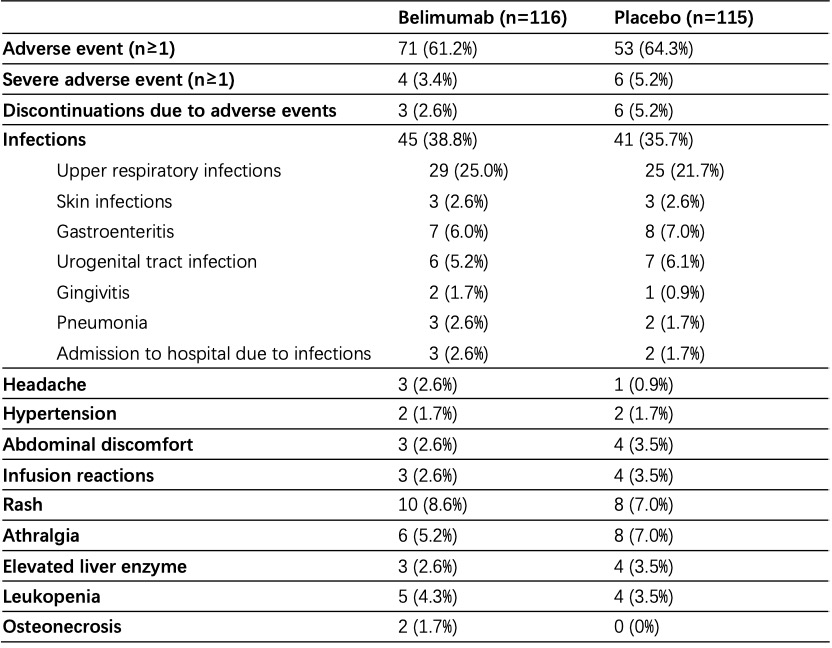
Table 2. Adverse events.
- FS
Abstract Poster Presenter(s)
Background/Purpose: B-lymphocyte stimulator was involved in the pathogenesis of SLE. The humanized monoclonal antibody belimumab with 10mg/kg was effective for active patients. The efficacy of low-dose belimumab for prevention of disease flares in patients with SLE with low disease activity was evaluated in this randomized trial (NCT04515719).
Methods: This was a 52-week, randomized, placebo-controlled trial. Patients who have Safety of Estrogens in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index (SELENA-SLEDAI) scores≤6; with no A score or no more than one B score on the British Isles Lupus Assessment Group (BILAG) scale; and who are treated with prednisone ≤20mg/day at screening were enrolled and randomly assigned in a 1:1 ratio to intravenous 120mg belimumab or placebo (saline) arm on weeks 0, 2, and 4, and then every 4 weeks until 48 weeks on the basis of standard of care (SOC). The primary outcome was a composite index of severe or mild-to-moderate disease flare (SELENA-SLEDAI Flare Index) within 52 weeks. Secondary outcomes included the percentage of severe flare, the percentage of mild-to-moderate flare, time to the first disease flare, changes in prednisone dose, SELENA-SLEDAI and safety analysis. The locked-down due to COVID-19 pandemic in Shanghai, China from April 2022 to June 2022 has greatly impacted patient recruitment and follow-up. Therefore, the trial was terminated prematurely at April 10th, 2022 when Renji Hospital, South campus was designated as a COVID-19 referral center during Shanghai locked-down.
Results: Overall, 90.5% of 116 patients receiving belimumab and 86.1% of 115 patients receiving placebo completed the study within a mean follow-up of 31.0 ± 16.1 weeks. Baseline characteristics were generally similar between treatment groups (Table 1). The primary endpoint was met. 7.8% (9/116) of patients receiving low-dose belimumab + SOC had disease flares within follow-up, which was significantly lower than that in patients receiving placebo + SOC, that is 19.1% (22/115) (p=0.012; difference 11.3%, OR 0.36 (95%CI 0.14~0.86); Figure 1A). Severe flares in belimumab group was only numerically lower than that in placebo group (1.7% vs 6.1%, p=0.1). Kaplan-Meier curves also demonstrated higher flare-free survival in patients receiving belimumab plus SOC (p=0.011, HR 2.63, 95% CI (1.21-5.72)) (Figure 1B). Glucocorticoid-sparing effects were observed in two groups (changes in prednisone from baseline to last visit, 1.64 ± 10.24 vs 0.91 ± 7.48, p=0.055). SELENA-SLEDAI was reduced by 0.62 ± 2.17 in belimumab group, however increased by 0.22 ± 2.82 in placebo group (p=0.004). Any adverse events were comparable between belimumab and placebo groups (61.2% vs 64.3%). Only 4 (3.4%) and 6 (5.2%) severe adverse events occurred in two groups (Table 2). There was no death.
Conclusion: Treatment with low-dose belimumab helped reduce disease flare risks in Chinese patients with SLE at low disease activity. Belimumab was generally well tolerated.

