Poster Session
Systemic Lupus Erythematosus (SLE)
Poster Session A
Session: (0641–0670) Systemic Lupus Erythematosus – Treatment Poster I
0642: Belimumab-Based Triple Therapy in Proliferative Lupus Nephritis: Renal Outcomes and Glucocorticoid Tapering in a Real-World Multicenter Cohort
Sunday, October 26, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
.jpg)
Table 1. Evolution of renal parameters over time
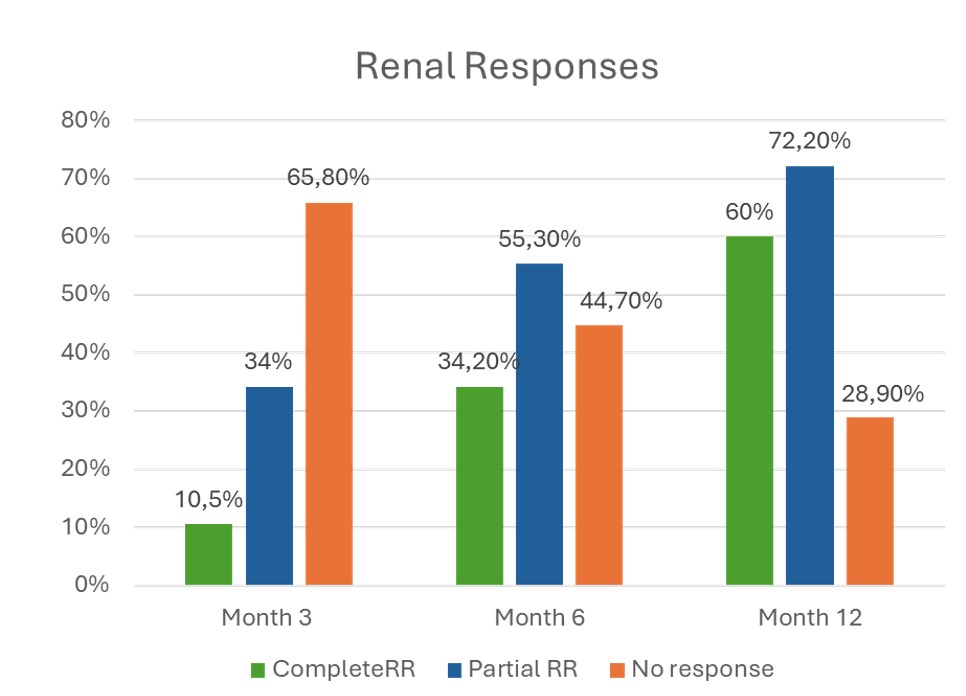
Figure 1. Evolution of Renal Response Rates
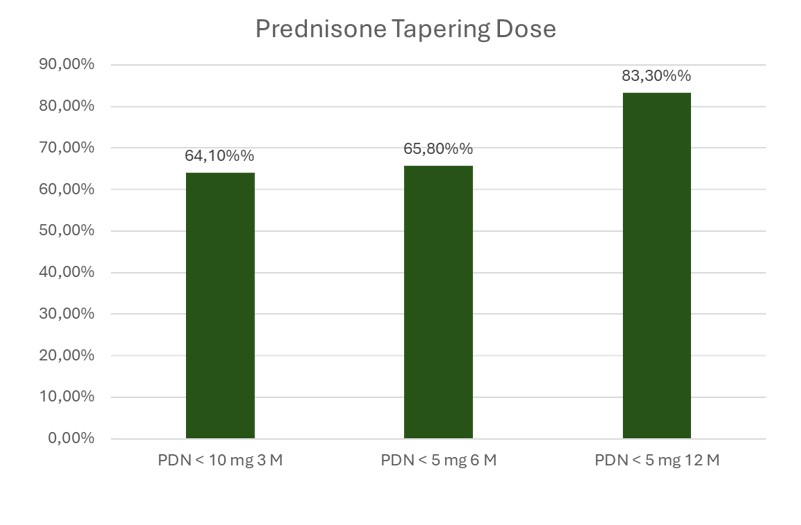
Figure 2. Proportion of Patients Achieving Glucocorticoid Tapering Targets
- JN
Javier Narvaez, PhD
Hospital Universitario de Bellvitge
Barcelona, SpainDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Background/Purpose: The newly published ACR guidelines recommend initiating triple therapy for all patients with proliferative lupus nephritis (LN). While belimumab (BEL) has demonstrated efficacy in clinical trials, real-world data on its early use in combination regimens remain limited.
We aimed to evaluate the effectiveness of triple therapy including BEL plus standard of care (SoC) initiated within 6 months of LN onset in achieving renal response and glucocorticoid tapering in a real-world multicenter cohort.
Methods: Retrospective multicenter study in Spain including patients with proliferative LN (ISN/RPS class III, IV, or mixed III/IV+V) treated with BEL-based triple therapy within 6 months of diagnosis.
Results: A total of 38 patients were included (78.9% female, mean age 38±14 years); 68.4% were Caucasian and 21.1% Hispanic. A history of previous LN episodes was present in 38.5% of patients.Histological classification included class III (n=11), class IV (n=20), and mixed III/IV+V (n=7); two had thrombotic microangiopathy. Mean activity and chronicity indices were 9.4±4.0 and 2.3±1.9, respectively. All patients had a minimum follow-up of 6 months, with a median follow-up of 25 months (IQR 14.5–41).
Induction therapy included intravenous methylprednisolone in 63.2%, with a mean initial oral prednisone dose of 35.7±17.9 mg/day. Most patients (76.3%) received MMF, while 23.7% were treated with cyclophosphamide. Maintenance therapy consisted of MMF in 94.7% of cases, with only two patients (5.3%) receiving azathioprine.
CRR was achieved in 60% within 12 months; 65.7% achieved a primary response and 72.2% a partial response (Figure 1). Proteinuria decreased from 3.0±2.9 g/day at baseline to 560±466 mg/day at 12 months; eGFR remained stable, with progressive improvement from 71.3±30.3 to 78.4±23.1 mL/min/1.73m² at 12 months and 80.9 at 24 months (Table 1).
Regarding glucocorticoid tapering, the 2023 ACR recommendations propose a target of ≤5 mg/day of prednisone by month 6. In our cohort, 65.8% of patients achieved this goal, with a mean dose of 6.0±3.2 mg/day at 6 months. Mean prednisone dose declined from 35.7±17.9 mg/day at baseline to 4.0±3.7 mg/day at 12 months. By month 12, 83.3% of patients were receiving ≤5 mg/day (Figure 2). At 24 months, mean prednisone was 3.6±5.0 mg/day (Table 1).
Renal relapse occurred in 6 patients while on treatment with BEL (15.7%); BEL was discontinued in 2, while 4 continued BEL with intensified immunosuppression. Three patients experienced mild to moderate non-renal flares, managed with therapy adjustments (anifrolumab, methotrexate, or prednisone increase).
When comparing early initiation of BEL ( < 3 months) versus delayed initiation (3–6 months post-flare), early use was associated with a significantly shorter time to achieve CRR (HR 4.7, 95% CI 1.6–13.5; p=0.04) and partial renal response (HR 2.47, 95% CI 1.1–5.8; p=0.037).
Conclusion: Initiation of triple therapy with BEL in proliferative LN was associated with robust renal responses and achievement of ACR steroid-sparing targets in most patients. Earlier treatment onset may further enhance renal outcomes

