Poster Session
Systemic Lupus Erythematosus (SLE)
Poster Session A
Session: (0641–0670) Systemic Lupus Erythematosus – Treatment Poster I
0647: anti-cd19 Chimeric Antigen Receptor T Cell Therapy for Refractory Systemic Lupus Erythematosus: An Open-label Pilot Study
Sunday, October 26, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
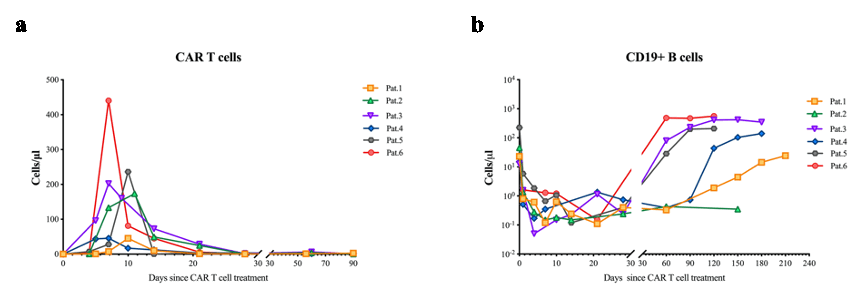
Fig.1 CAR T cell expansion in vivo and depletion of B cells.
a, Circulating CAR T cell number in the six patients within the 90 days after treatment (N=6). b, Circulating B cell numbers in the six patients since treatment (N=6).
a, Circulating CAR T cell number in the six patients within the 90 days after treatment (N=6). b, Circulating B cell numbers in the six patients since treatment (N=6).
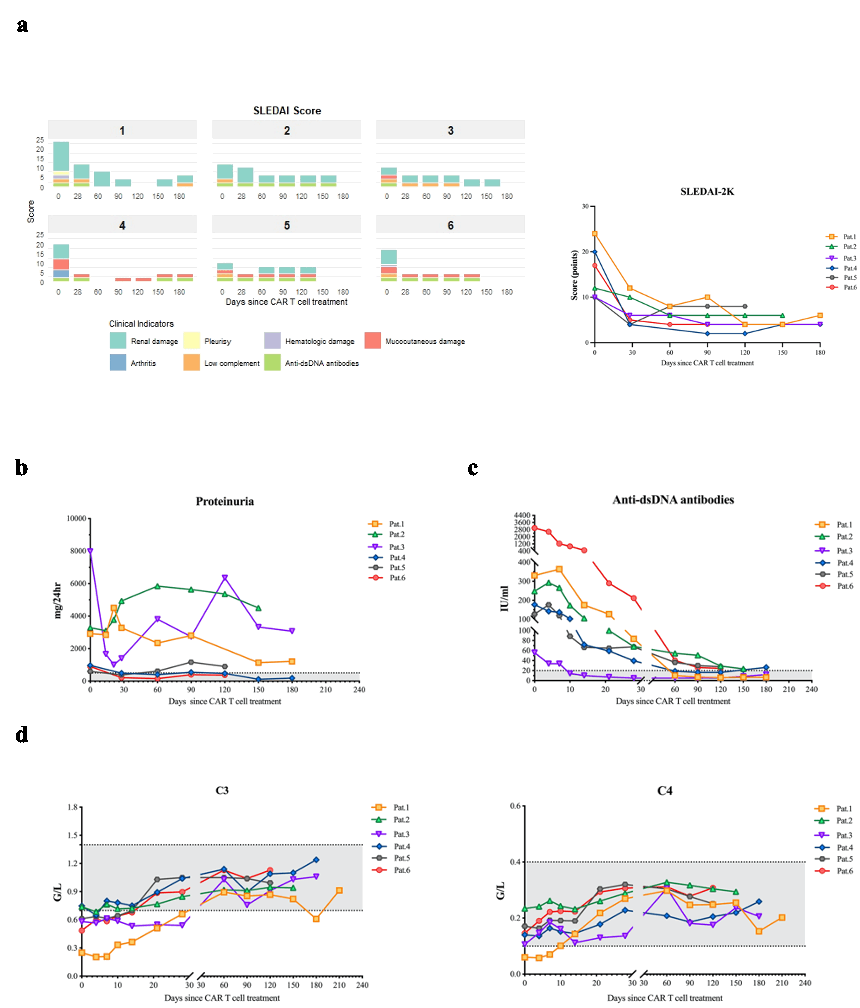
Fig.2 Effects of CAR T cell treatment on the activity of systemic lupus erythematosus.
a, The change of SLEDAI-2K scores after CAR T cell administration (N=6). b, The change of proteinuria after CAR T cell administration (N=6). c, Anti-double stranded (ds) DNA antibodies assessed by radioimmunoassay after CAR T cell administration (N=6).d, The change of complement factor C3 and C4 levels after CAR T cell administration (N=6).
The shaded gray areas in b through d indicate the normal range.
SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2K.
a, The change of SLEDAI-2K scores after CAR T cell administration (N=6). b, The change of proteinuria after CAR T cell administration (N=6). c, Anti-double stranded (ds) DNA antibodies assessed by radioimmunoassay after CAR T cell administration (N=6).d, The change of complement factor C3 and C4 levels after CAR T cell administration (N=6).
The shaded gray areas in b through d indicate the normal range.
SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index-2K.
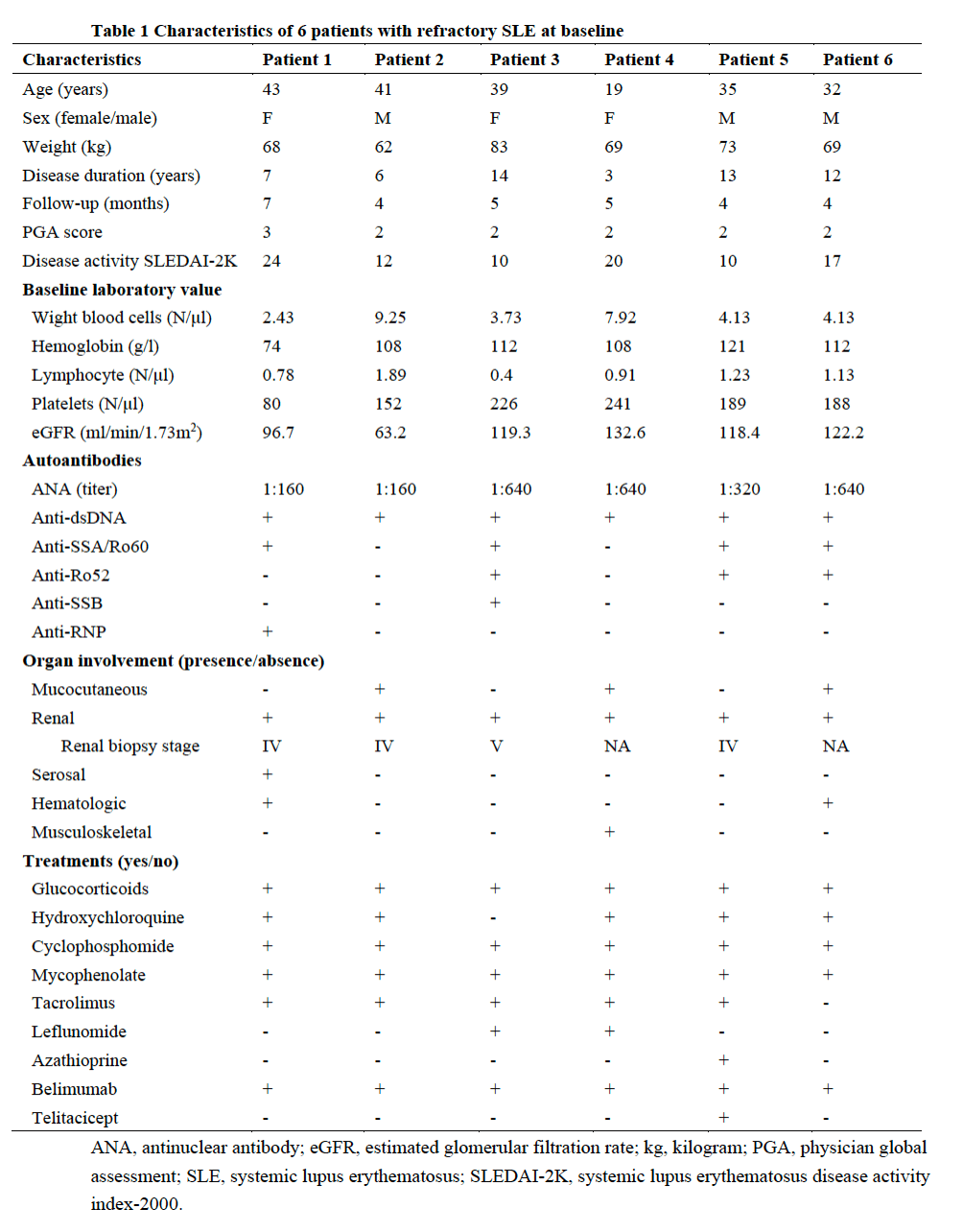
Table 1 Characteristics of 6 patients with refractory SLE at baseline
- RM
Rong Mu, n/a
Peking University Third Hospital
Beijing, China (People's Republic)Disclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: This pilot study aims to evaluate the preliminary efficacy and safety of autologous anti-CD19 chimeric antigen receptor T cell (CAR T) named IM19 therapy in patients with refractory systemic lupus erythematosus (SLE).
Methods: Six patients with refractory SLE (three women and three men) with a median (range) age of 37 (19 to 43) years, median (range) disease duration of 9.5 (3 to 14) years were enrolled in the study. All met the 2019 EULAR/ACR classification criteria for SLE and had active disease (SLEDAI-2K≥10) with inadequate response to at least two lines of therapies including steroids, immunosuppressants and biologic agents. Autologous T cells from patients with SLE were transduced with a lentiviral anti-CD19 CAR vector, expanded and reinfused at a dose of 1×106 CAR T cells per kg body weight or 1 ×108 cells into the patients after lymphodepletion with fludarabine and cyclophosphamide. Preliminary efficacy was assessed by the changes of SLEDAI-2K at 3 months after CAR T cell therapy. Safety was assessed by recording cytokine-release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) during the first 28 days. The study was registered at ClinicalTrials.gov (NCT06513429).
Results: The demographic and clinical characteristics of the patient at baseline are summarized in Table 1. The median (range) follow-up was 5.5 (4 to 7) months. The CAR T cells expanded significantly (median peak CAR+ T cells: 188.1cells/μL at median day 8.5 (range 7-11))(Fig.1 a). Complete circulating B-cell depletion (CD19+ B cells < 5 cells/μL) was achieved in 5/6 patients by day 1 post-infusion and in all patients by day 4 (Fig.1 b). The median SLEDAI-2K score demonstrated a significant reduction from 12 (range, 10 to 24) to 5.5 (range, 4 to 13) at 1 month, with a further decline to 5.0 (range, 2 to 10) by the 3-month follow up (Fig.2 a). During the observation period, complete renal remission was achieved in 2 patients, with partial remission observed in 1 patient. The remaining 3 patients exhibited persistent proteinuria with no significant improvement throughout the follow-up period (Fig.2 b). Anti-dsDNA antibodies converted to negative in 3 patients (Fig.2 c) and the complement returned to normal levels in all the patients within 2 months after CAR T cell administration (Fig.2 d). IM19 CAR T cell therapy was well tolerant with grade 1 CRS in 4 patients. No ICANS occurred in the study.
Conclusion: IM19 CAR T cell therapy demonstrated an acceptable safety profile and induced profound circulating B-cell depletion in refractory SLE, which correlated with accelerated serological normalization and clinically meaningful reductions in disease activity. Notably, the study revealed renal response in 50% of participants with refractory lupus nephritis, indicating a selective rather than universal therapeutic effect. These findings underscore the necessity of future large-scale trials with prolonged follow-up durations to evaluate the durability of therapeutic effects and safety profiles of this innovative cellular therapy.

