Poster Session
Systemic Lupus Erythematosus (SLE)
Poster Session B
Session: (1517–1552) Systemic Lupus Erythematosus – Treatment Poster II
1529: Updated Phase 1 Trial Data Assessing the Tolerability, Efficacy, Pharmacokinetics, and Pharmacodynamics of BMS-986353 (CC-97540), a CD19-directed Chimeric Antigen Receptor T Cell Therapy Using a Next-Generation Process for Severe, Refractory SLE
Monday, October 27, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
.jpg)
CRS and ICANS at Part B expansion dose.
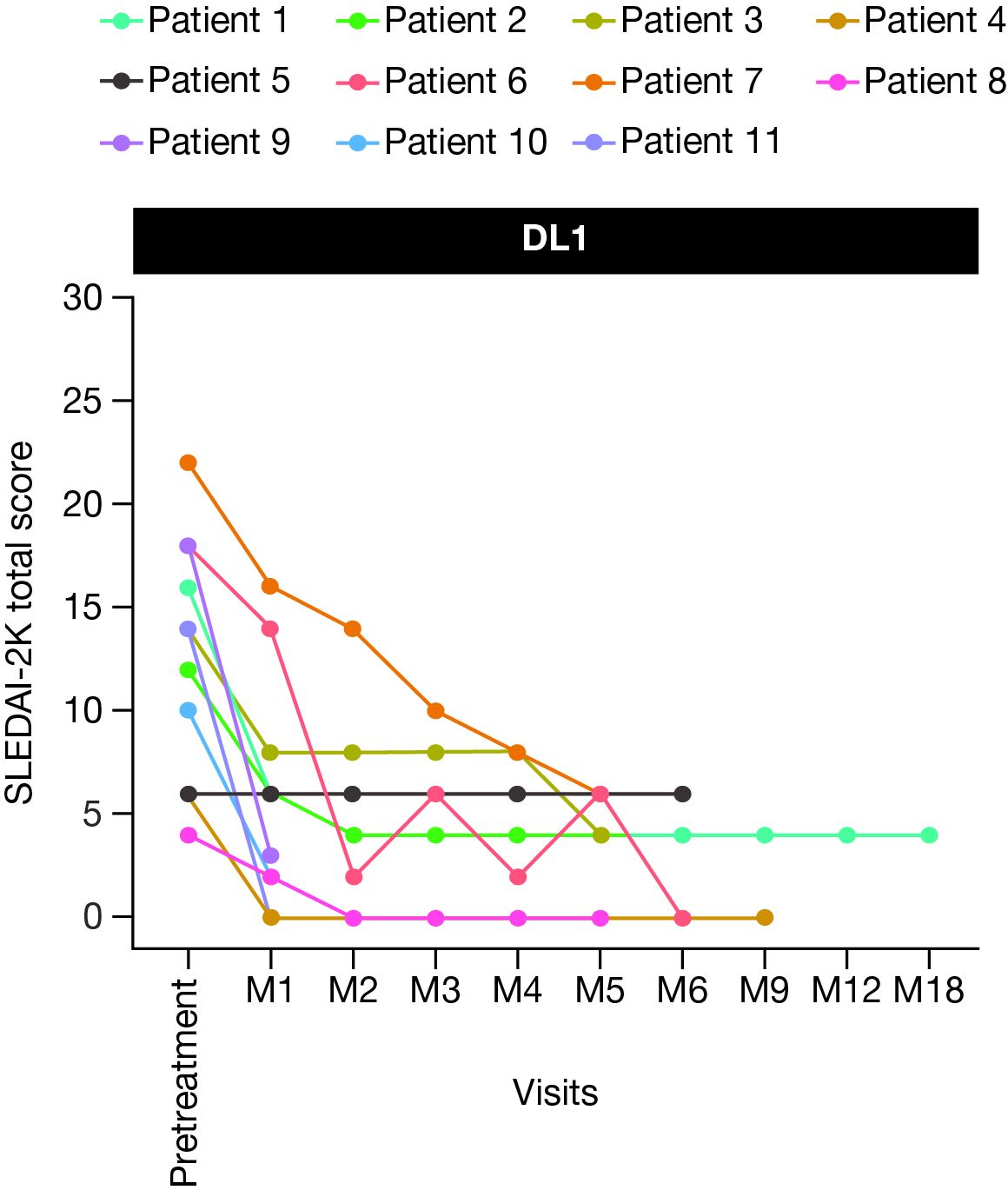
Figure 1. Total SLEDAI-2K score following BMS-986353 treatment in the SLE efficacy-evaluable population at DL1.
DL, dose level; M, month; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
DL, dose level; M, month; SLE, systemic lupus erythematosus; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000.
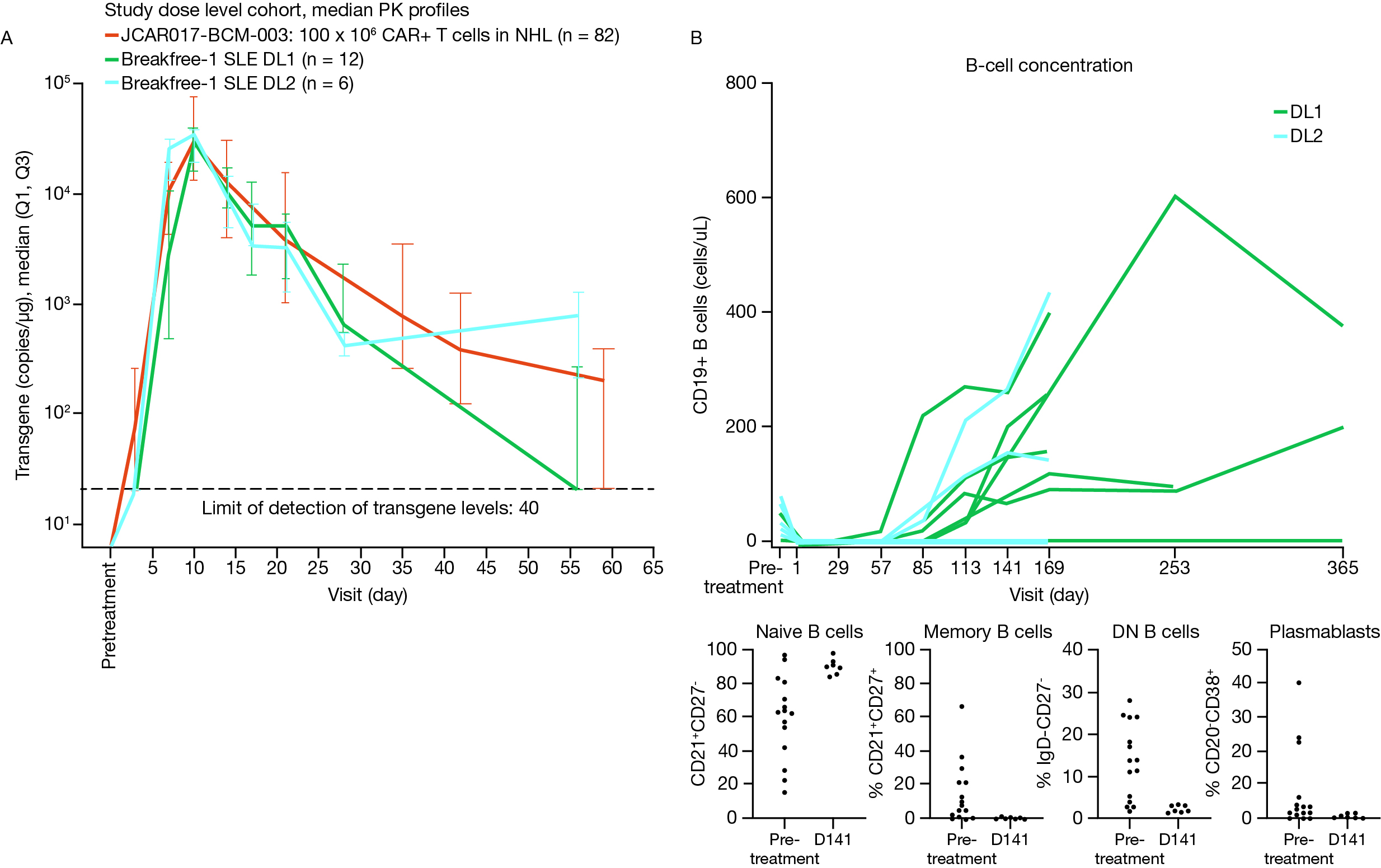
Figure 2. Median transgene PK profiles (A) and peripheral B cell concentration and phenotype (B) following BMS-986353 treatment in the SLE population.
PK were analyzed using droplet PCR and pharmacodynamics were evaluated via flow cytometry.
DL, dose level; DN, double negative; PCR, polymerase chain reaction; PK, pharmacokinetics; SLE, systemic lupus erythematosus.
PK were analyzed using droplet PCR and pharmacodynamics were evaluated via flow cytometry.
DL, dose level; DN, double negative; PCR, polymerase chain reaction; PK, pharmacokinetics; SLE, systemic lupus erythematosus.

Georg Schett, MD
Vice President Research
Uniklinikum Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany
Erlangen, GermanyDisclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: BMS-986353 (CC-97540; CD19 NEX-T) is a CD19-directed chimeric antigen receptor (CAR) T cell therapy that expresses the same CAR as lisocabtagene maraleucel (liso-cel); it is manufactured via the NEX-T™ process to reduce manufacturing time and optimize phenotypic attributes. In Breakfree-1 trial (NCT05869955), BMS-986353 showed promising initial safety and efficacy in pts with severe refractory SLE.1 Here, we report updated data on BMS-986353 in the SLE cohort.
Methods: This phase 1, multicenter trial assesses safety and efficacy of BMS-986353 in pts with severe, treatment-resistant autoimmune diseases. Inclusion criteria required active disease (BILAG A score) and an inadequate response to ≥ 2 immunosuppressants and steroids. After lymphodepletion, a single BMS-986353 infusion was administered at 2 dose levels (DLs) to optimize risk–benefit in SLE: DL1, 10×106; DL2, 25×106 CAR+ T cells. All pts were weaned off lupus-directed therapies by day 29 post-infusion. Primary endpoint was safety.
Results: As of March 17, 2025, 26 pts with SLE were enrolled; 19 were treated with BMS-986353 (DL1, n=13; DL2, n=6), with 17 (DL1, n=11) evaluable for efficacy. Median age: 30.0 (18–49) years. Median follow-up (range): 181.0 (7–488) days. Four and 15 pts had BILAG A cardiorespiratory and BILAG A renal involvement, respectively, and had refractory disease despite multiple prior therapies (median [range]: 7.0 [4–11]).
Lower frequency and grade (gr) of adverse events were observed in DL1 vs DL2. Gr 3/4 neutropenia occurred in 46.2% (DL1) versus 83.3% (DL2); all events resolved. There were no gr 3/4 thrombocytopenia or prolonged cytopenias at either DL. Low gr cytokine release syndrome (CRS) occurred in 61.5% at DL1 (Table) versus 100% at DL2; no gr ≥3 CRS events were observed at either DL. Immune effector cell-associated neurotoxicity syndrome (ICANS) was not observed at DL1; 2 gr 3 ICANS events occurred at DL2. All CRS and ICANS were brief and reversible. DL1 was selected for expansion in Part B based on the improved safety profile at DL1 in Part A.
After BMS-986353 administration, most evaluable pts experienced a substantial improvement in disease activity as assessed by a significant reduction in SLEDAI-2000K (2K) and Physician’s Global Assessment (PGA) scores; with 6 months of follow-up, DL1 pts had a median 9 point reduction in SLEDAI-2K score (Fig 1) and a 90% improvement in PGA. No clinically meaningful differences in efficacy outcomes were observed between DLs.
Double-stranded DNA and serum complement (C3 or C4) had significant titer reduction or normalized over time for pts with abnormal values at screening. All pts showed robust CAR T cell expansion compared with liso-cel and complete peripheral blood B-cell depletion at both DLs with primarily naive repopulating B cells (Fig 2).
Conclusion: These data show a manageable safety profile and preliminary clinical benefit of BMS-986353 for pts with severe, treatment-resistant SLE, suggesting BMS-986353 may be a potential therapeutic option.
Reference: 1. Schett G, et al. Arthritis Rheumatol 2024;76(suppl 9).
Medical writing: Alexus Shirk, PhD (Caudex, an IPG Health Company), funded by Bristol Myers Squibb.

