Poster Session
Hematologic and Oncologic Associated Rheumatic Syndromes
Poster Session B
Session: (1088–1122) Immunological Complications of Medical Therapy Poster
1108: Baseline Features, Immunosuppression, and Immune-related Adverse Events in Patients with Pre-existing Rheumatic Disease and Cancer Requiring Immune Checkpoint Inhibitor Therapy
Monday, October 27, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
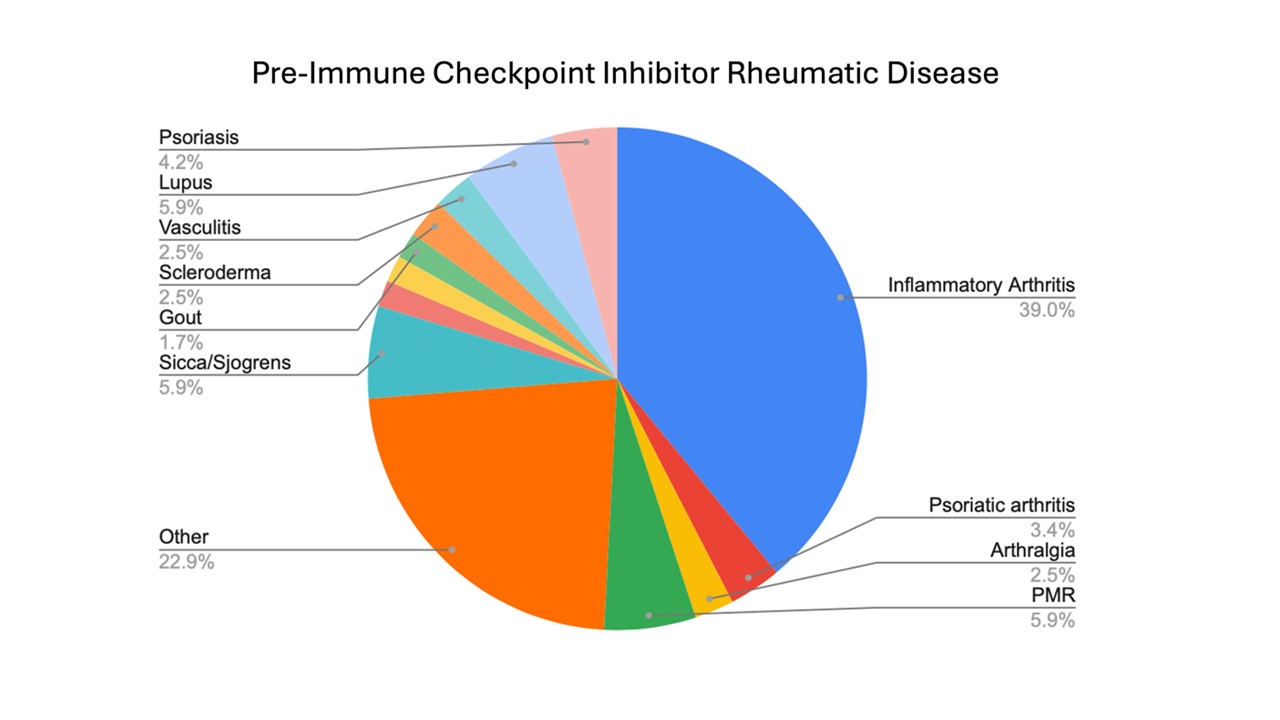
Figure 1: Frequency of Pre-Immune Checkpoint Inhibitor Rheumatic Diseases in the RADIOS Study
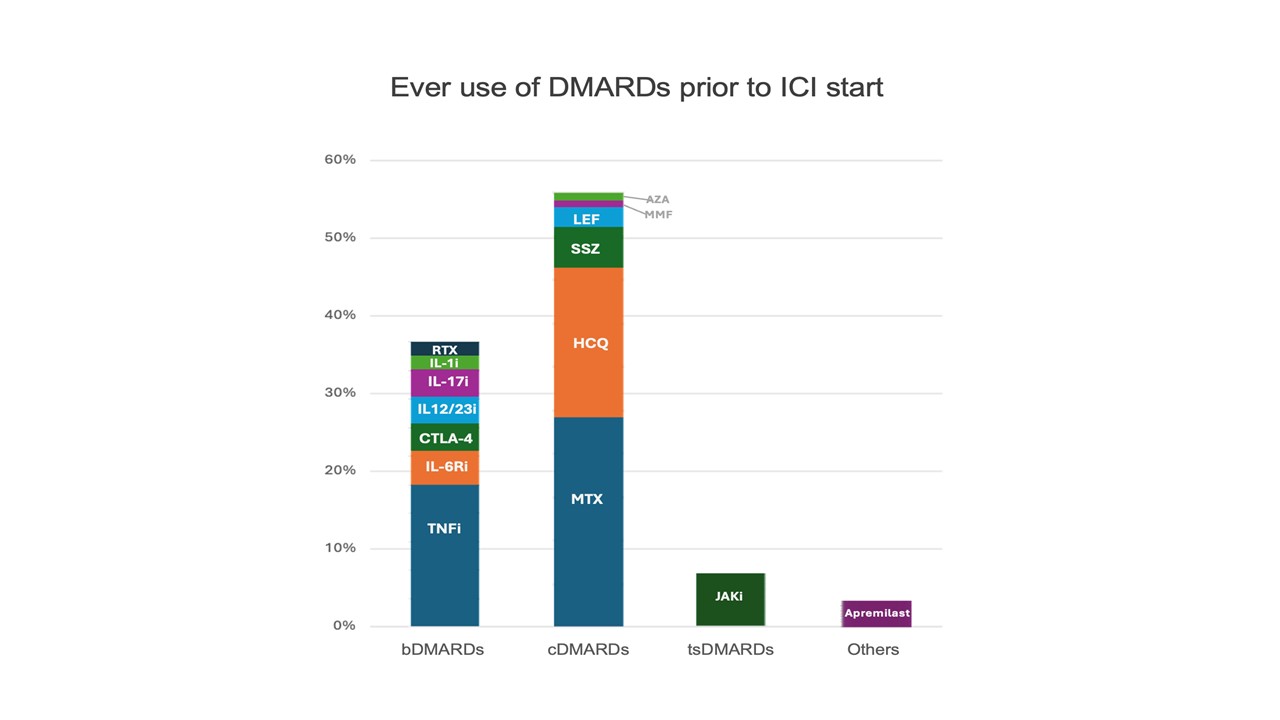
Figure 2: Ever use of disease modifying anti-rheumatic drugs (DMARDs) prior to immune checkpoint inhibitor (ICI) start
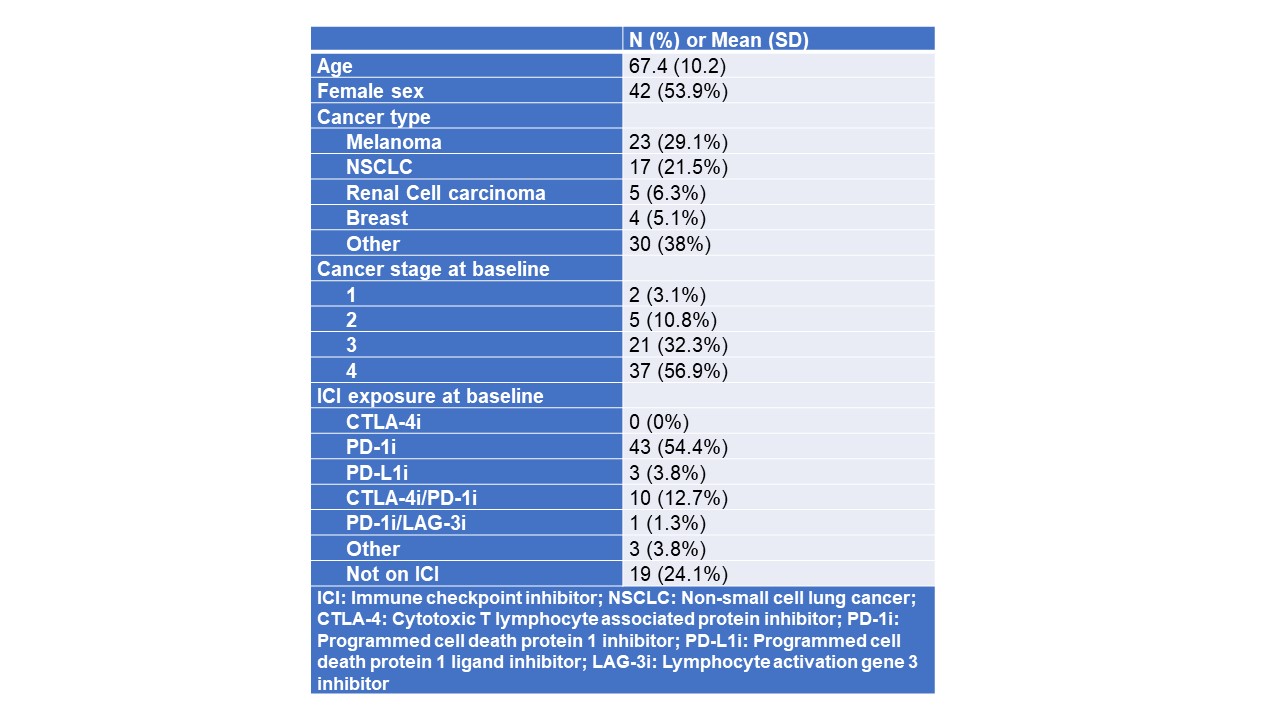
Table 1: Demographics and baseline characteristics for patients with pre-ICI Rheumatic Disease
.png)
Laura Cappelli, MD
Johns Hopkins School of Medicine
Baltimore, MD, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: Immune checkpoint inhibitors (ICIs) are effective therapies commonly used to treat malignancies but can cause flares or unrelated immune-related adverse events (irAEs) in patients with pre-existing rheumatic disease prior to ICI initiation (pre-ICI RD). As patients with pre-ICI RD were excluded from initial ICI studies, there is still a paucity of information about risks of ICI treatment and how to manage pre-ICI RD while patients are on ICIs.
Methods: Rheumatic Adverse Events Due to Cancer Immunotherapy Observational Studies (RADIOS) is a United States-based academic consortium of 12 sites prospectively collecting data to evaluate outcomes of patients with pre-ICI RD who receive cancer immunotherapy. Patients were included if they had pre-ICI RD and had a baseline visit with rheumatology. Descriptive statistics were calculated for the cohort’s demographics (including age, sex, race, ethnicity), cancer characteristics, ICI use at enrollment, and prior treatment for autoimmune disease; clinical disease activity index (CDAI) was calculated for those with inflammatory arthritis. Data on irAE development and autoimmune disease treatment were queried for follow up visits.
Results: As of 4/1/2025, 78 patients with pre-ICI RD were enrolled into the cohort; mean age was 67 years and 54% were female. Inflammatory arthritis was the most common diagnosis (39%) followed by systemic lupus (5.9%) and Sjogren’s disease (5.9%) (Figure 1). Melanoma was the most common cancer type followed by non-small cell lung cancer. The majority of patients had stage 4 cancer, and anti-PD-1 was the most common category of ICI used (Table 1). Patients had been previously treated for their RD with a wide variety of DMARDs, most commonly TNF-inhibitors, methotrexate, and hydroxychloroquine (Figure 2). At RADIOS enrollment, 23 (29.5%) were currently on corticosteroids. For those with inflammatory arthritis and CDAI available (n=36), mean CDAI was 11.4 (SD 11.4) and most had low disease activity or remission (n=20, 55.6%). The most common non-rheumatic irAEs developed were rash and pneumonitis (n=5 for both). At follow up visits, TNF-inhibitors were the most administered biologics (n=9) followed by IL-6R inhibitors (n=7); methotrexate (n=15) and hydroxychloroquine (n=13) were the most common conventional synthetic DMARDs administered. No patients received JAK-inhibitors in follow-up.
Conclusion: By characterizing treatment patterns and outcomes in diverse rheumatic diseases, this multi-institutional, prospective research collaboration will improve knowledge regarding ICI safety for patients with rheumatic diseases who require ICI therapy for cancer.

