Poster Session
Pain Syndromes, Fibromyalgia, and Regional Musculoskeletal Disorders
Poster Session B
Session: (1221–1247) Pain in Rheumatic Disease Including Fibromyalgia Poster
1231: TNX-102 SL, Cyclobenzaprine HCl Sublingual Tablets, Demonstrates Pain Reduction and Favorable Tolerability in Patients with Fibromyalgia
Monday, October 27, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
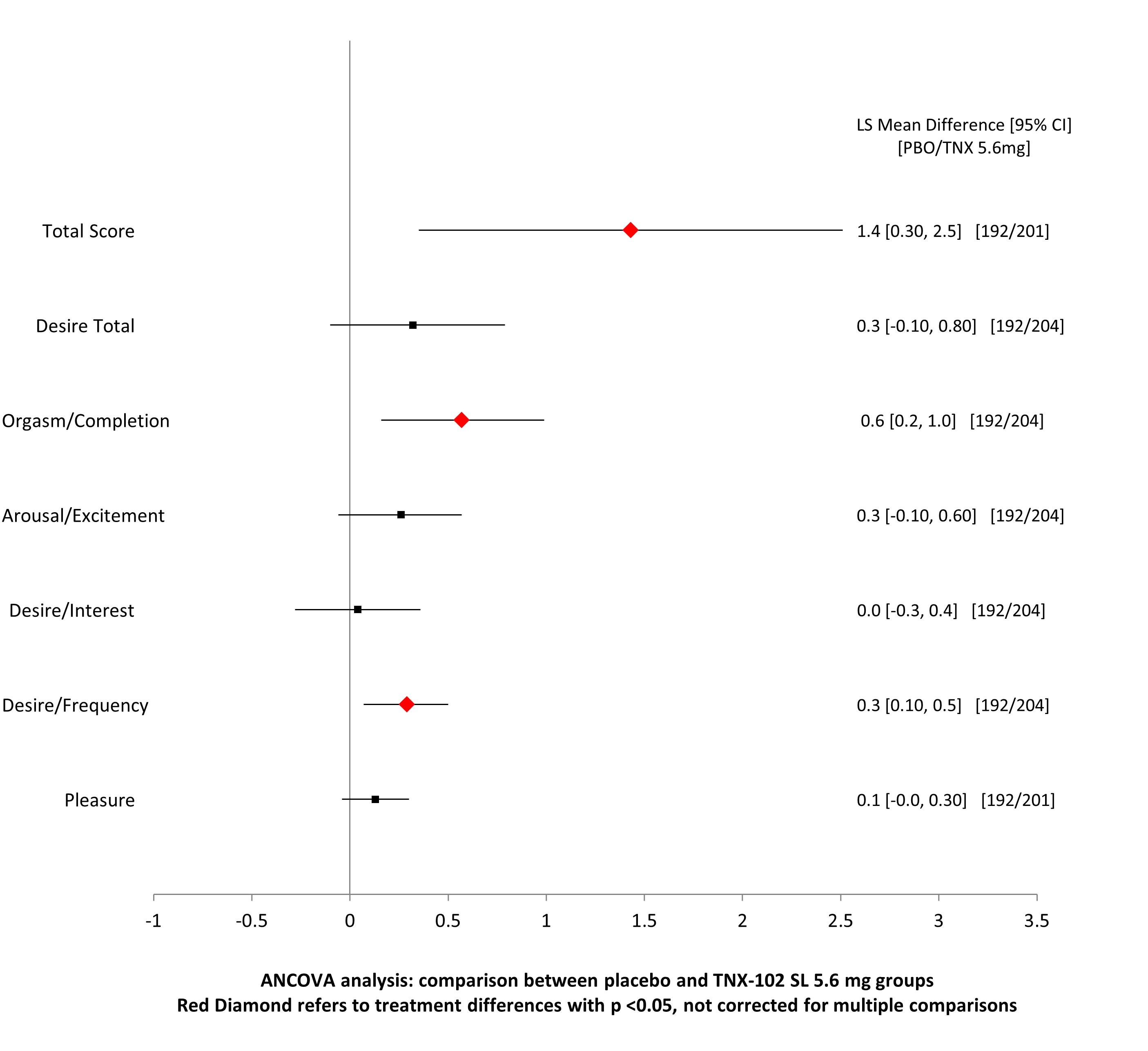
Changes in Sexual Functioning Questionnaire Change from Baseline to Week 14 in Females
.png)
Gregory Sullivan, MD
Tonix Pharmaceuticals Inc
Chatham, NJ, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: FDA-approved treatments for fibromyalgia (FM) have historically been limited by intolerable side effects that often lead to poor adherence. TNX-102 SL, a sublingual formulation of cyclobenzaprine, is under evaluation by the FDA as a treatment for FM. TNX-102 SL treatment has shown statistically significant reduction in pain in two Phase 3 studies, including the RESILIENT study reported below. In these studies, TNX-102 SL was generally well tolerated. The tolerability of TNX-102 SL may provide an appealing alternative to existing therapies.
Methods: The RESILIENT study was a 14-week, randomized, double-blind, placebo-controlled trial designed to evaluate the efficacy and safety of TNX-102 SL in patients with FM. The pre-specified primary endpoint was the change from baseline to Week 14 in the weekly average of daily pain numeric rating scale (NRS) scores, analyzed using a mixed model for repeated measures with multiple imputation for missing data. Exploratory secondary endpoints included assessments of tolerability, such as changes in sexual function (measured by CSFQ-14) and weight gain. Safety evaluations included changes in blood pressure and the incidence of adverse events (AEs).
Results: TNX-102 SL demonstrated significant improvement in the primary pain endpoint compared to placebo (p < 0.0001) at Week 14. Females treated with TNX-102 SL had significantly greater improvements in desire/frequency (p = 0.010), orgasm/completion (p = 0.007), and total sexual function scores (p = 0.010). No significant differences were observed between TNX-102 SL and placebo in weight, systolic, or diastolic blood pressure at Week 14. The most commonly reported AEs were mild to moderate, self-limited events such as transient tongue or mouth numbness and bitter aftertaste, which rarely led to study discontinuation. Systemic AE rates, excluding COVID-19, remained below 4.0%.
Conclusion: TNX-102 SL significantly reduced pain and showed a favorable tolerability profile, including minimal impact on weight and blood pressure, along with its unique mechanism targeting sleep disturbances, supports its potential as a new treatment option for FM. The availability of a well-tolerated treatment may also encourage clinicians to make the diagnosis of fibromyalgia earlier, thereby improving patient outcomes through timely intervention.

