Poster Session
Spondyloarthritis (SpA) Including Psoriatic Arthritis (PsA)
Poster Session C
Session: (2338–2376) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
2371: A Predictive Model Combining Clinical and Laboratory Parameters to Predict Anti-tnf Response in Patients with Psoriatic Arthritis
Tuesday, October 28, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
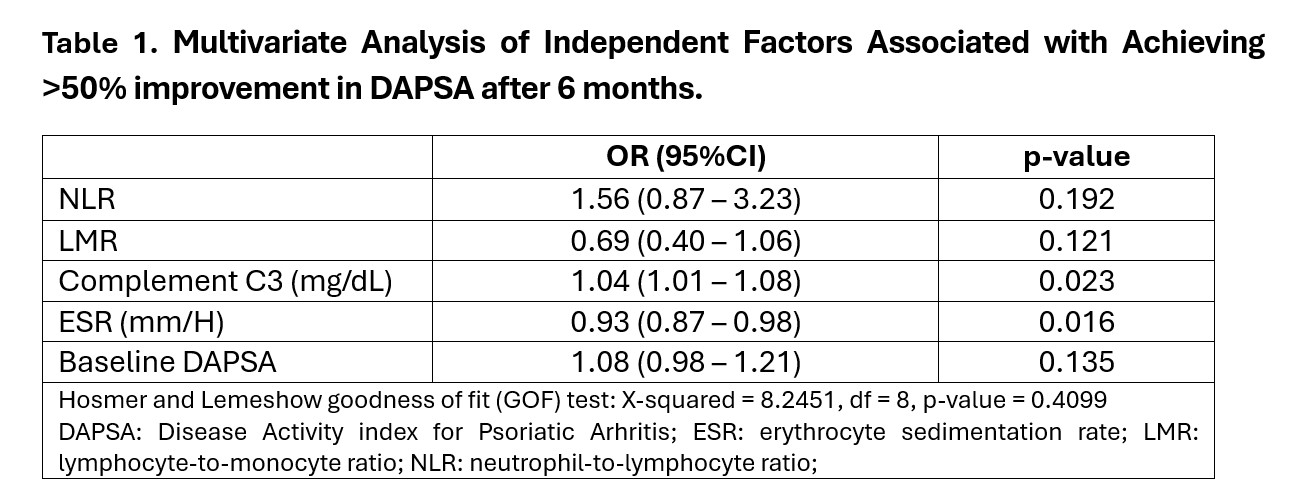
Table 1. Multivariate Analysis of Independent Factors Associated with Achieving >50% improvement in DAPSA after 6 months.
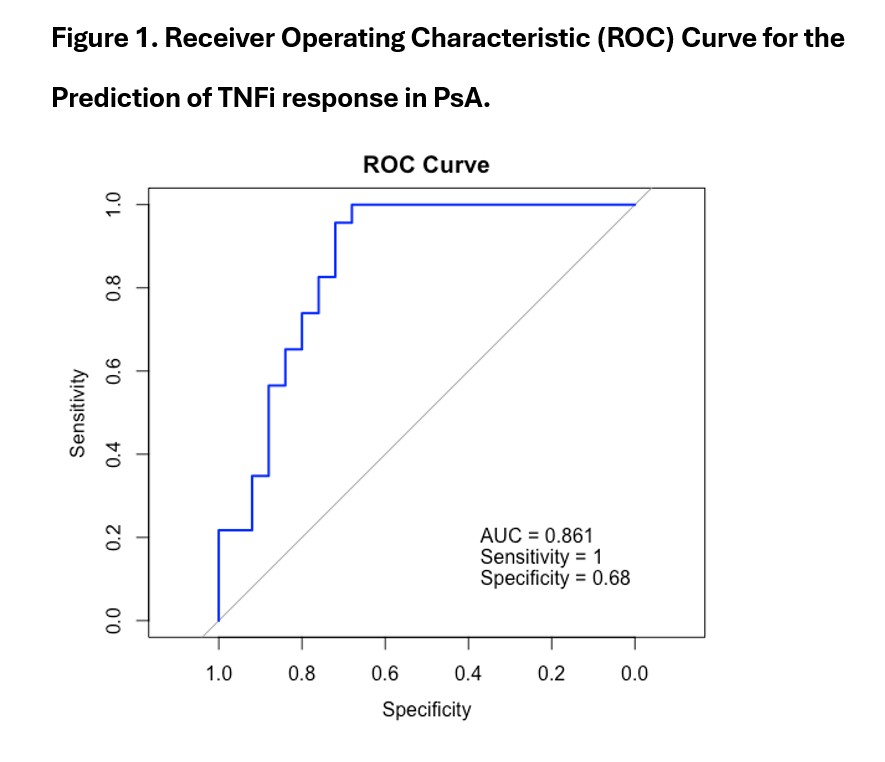
Figure 1. Receiver Operating Characteristic (ROC) Curve for the Prediction of TNFi response in PsA.
- RG
Raquel Granados, MD
Hospital Universitario Reina Sofia
Cordoba, SpainDisclosure(s): No financial relationships with ineligible companies to disclose
Abstract Poster Presenter(s)
Background/Purpose: Psoriatic arthritis (PsA) is a heterogeneous inflammatory disease requiring individualized treatment strategies. Early identification of patients likely to respond to therapy is essential to optimize outcomes and avoid unnecessary treatment exposure. Hematologic and inflammatory biomarkers, readily available in clinical practice, may offer predictive value in guiding treatment decisions. In this study, we aimed to identify a combination of clinical data and routinely available laboratory markers capable of reliably predicting which patients with PsA will respond to TNF inhibitors (TNFi).
Methods: This was a prospective analysis including 68 patients diagnosed PsA who fulfilled the CASPAR criteria. A multivariable logistic regression model was initially constructed using routinely measured inflammatory and hematologic parameters, with Disease Activity index for Psoriatic Arhritis (DAPSA) response as the dependent variable, defined as an improvement of >50% in the DAPSA score. To optimize model simplicity and performance, stepwise variable selection was applied in both forward and backward directions, based on the Akaike Information Criterion (AIC). This method iteratively adds or removes predictors to identify the model with the best balance between goodness-of-fit and parsimony. Model performance was evaluated using classification accuracy and the area under the ROC curve (AUC).
Results: A total of 68 patients were included, 55.8% of whom were male, with a mean age of 50 years (SD 16.7) and a mean disease duration of 7.2 years. A total of 31 patients (45.6%) responded to TNFi. The multivariable analysis (Table 1) identified five independent factors associated with achieving >50% improvement in DAPSA after 6 months: neutrophil-to-lymphocyte ratio (NLR) (OR 1.56), lymphocyte-to-monocyte ratio (LMR) (OR 0.69), complement C3 (OR 1.04), erythrocyte sedimentation rate (ESR) (OR 0.93), and baseline DAPSA score (OR 1.08). The final model demonstrated excellent discrimination, with an AUC of 0.861 (95% CI: 0.752–0.970) (Figure 1). It correctly classified 100% of the responders (sensitivity = 1.00) and 68% of the non-responders (specificity = 0.68).
Conclusion: A combination of routinely available hematologic and inflammatory markers can effectively predict clinical response to TNFi in patients with PsA, but further validation of this model is needed in independent cohorts. This predictive model may support more personalized and efficient treatment decisions in routine clinical practice.

