Poster Session
Rheumatoid Arthritis (RA)
Poster Session B
Session: (1347–1375) Rheumatoid Arthritis – Treatment Poster II
1355: Use of parenteral compared to oral glucocorticoids in early rheumatoid arthritis is superior for chance ofbeing off steroids and escalation of therapy at 1 year
Monday, October 27, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
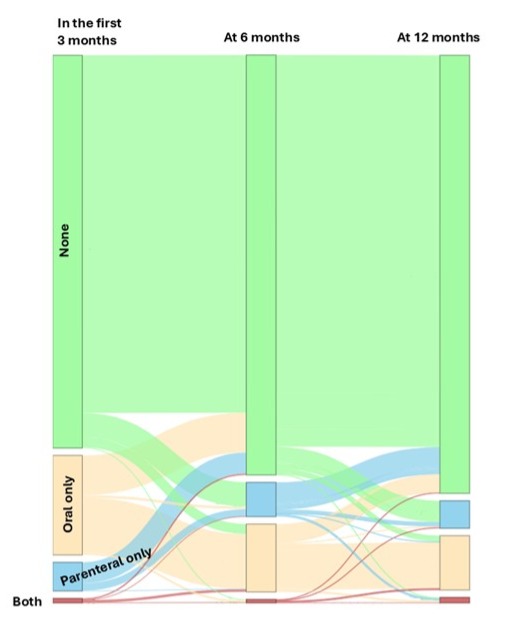
Table 1. Multivariate logistic regression estimating the odds for GC and advanced therapy use over time

Janet Pope, MD, FRCPC, MPH
University of Western Ontario
London, Ontario, CanadaDisclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: The 2023 EULAR recommendations for the management of Rheumatoid Arthritis (RA) emphasizes the
importance of limiting the dose and duration of glucocorticoids (GC) used in early disease. However, no
recommendation about the preferred route of GC administration is available, nor is it known if GC route is
associated with total GC exposure. In this study we describe the route of GC administration in patients with early RA (ERA), and determine if GC route is associated with the likelihood of being GC-free and/or requiring advanced therapy at 12 months.
Methods: Participants included newly diagnosed RA patients (symptoms < 1 year) enrolled in the Canadian Early Arthritis
Cohort (CATCH) between (dates) and excluded if they reported GC use 90 days prior to baseline, were on
advanced therapy by 3 months, or had < 12 months. Patients were stratified by use of GC during the first 3
months of follow-up: none; oral only; parenteral only (intramuscular or intraarticular); or both (oral
+parenteral). Multivariate logistic regression adjusted by confounders was used to calculate the OR of GC use
at 6 and 12 months and/or progression to advanced therapies (biologics or JAKi).
Results: The sample included 2,222 ERA patients. Mean (SD) age was 55 (15), disease duration 5.5 (3) months, 73%
were female, and 86% were white, 69% were initially on methotrexate (MTX) at a mean dose of 20.1 (4.2)
mg/week. Mean CDAI at baseline and 12-month scores were 26 (14) and 7.5 (8.6).
The majority (1,661; 75%) received no GC; oral-only GC was 421 (19%); parenteral-only GC was 121 (5%), and
both oral and parenteral was 19 (1%). Mean CDAI at baseline was lowest in the no GC and highest in those
receiving both (24.1 vs 31.6, vs 30.2, vs 32.8, p< 0.0001), but no differences were observed among groups at 12
months. GC and advanced therapeutics at 12 months were: 8% and 7% (no GC); 47% and 14% (oral); 26% and
14% (parenteral); 63% and 16% (both). Table 1 shows that any GC use, particularly oral-only, increases odds of
chronic GC use at 6 and 12 months, as well as advanced therapy utilization. Figure 1 features a Sankey diagram
illustrating changes in GC use over 12 months.
Conclusion: Initial use of GC is low despite EULAR recommendations suggesting their use for patients with higher baseline
disease activity. Among patients with active early RA, those receiving parenteral GC were half as likely to
remain on GC at 12 months, compared to those using oral GC. Both GC groups had similar rates of use of
advanced treatment use, which were higher compared to the no GC group. Parenteral GC use may support
earlier discontinuation of steroids.

