Poster Session
Scleroderma
Poster Session B
Session: (0955–0977) Systemic Sclerosis & Related Disorders – Basic Science Poster I
0972: Prominent endothelial senescence in systemic sclerosis skin
Monday, October 27, 2025
10:30 AM - 12:30 PM Central Time
Location: Hall F1
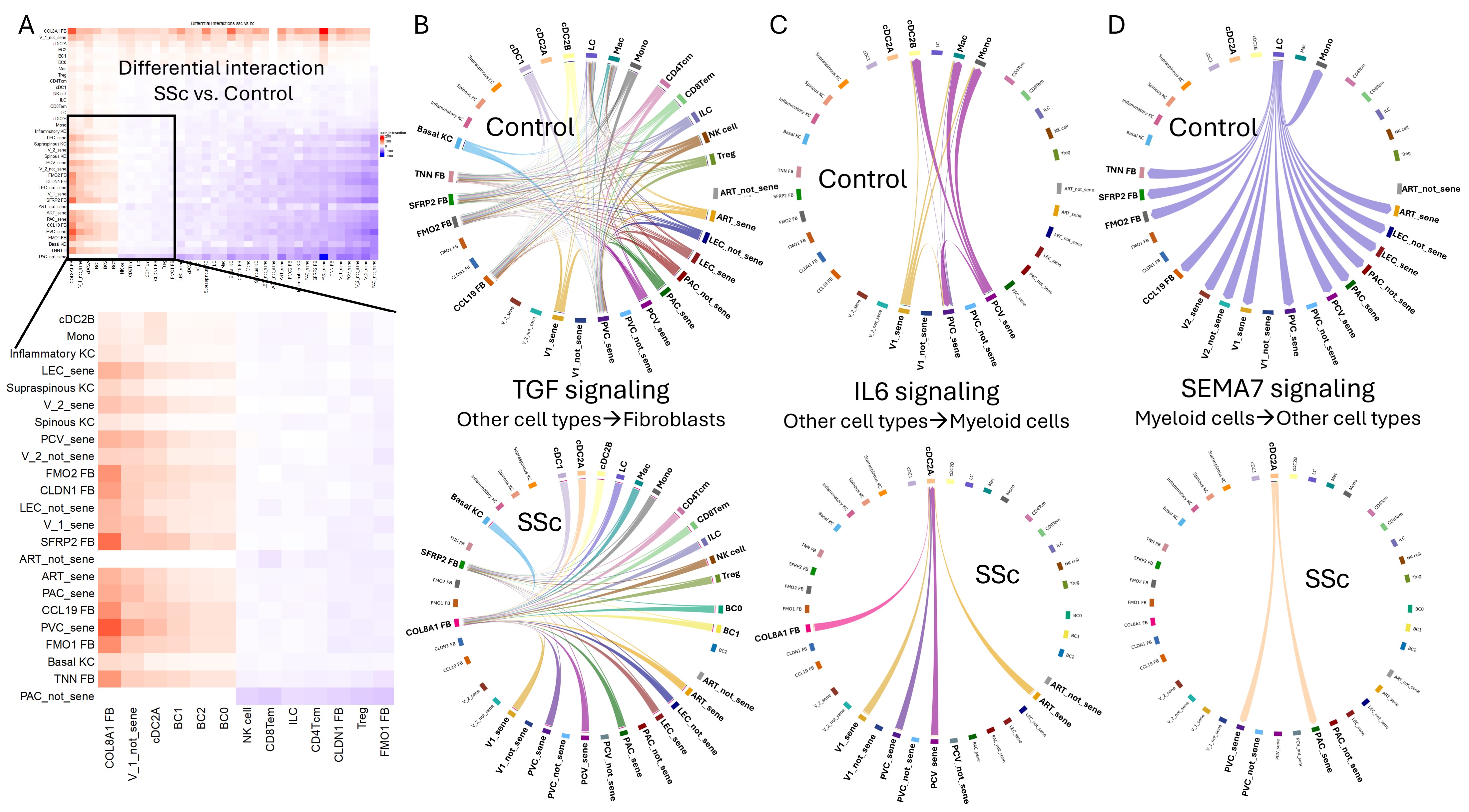
Figure 1. Bioinformatics analysis reveals that senescent SSc ECs engage in unique signaling interactions with myofibroblasts and DCs. Receptor-ligand interaction analysis, comparing SSc skin to controls, shows that senescent EC clusters have the strongest interactions with COL8A1+ myofibroblasts, cDC2A LAMP3+ DCs cells, and B cells, whereas non-senescent ECs exhibit minimal interactions (A). TGFβ signaling targeting COL8A1+ myofibroblasts in SSc skin originates predominantly from senescent ECs, among other cell types, unlike non-senescent ECs (B). In SSc skin, IL-6 signaling to LAMP3+ DCs is contributed by both COL8A1+ myofibroblasts and senescent ECs (C). Senescent PVC and PAC EC clusters interact with LAMP3+ DCs through SEMA7-integrin binding (D). ART: arterial, PAC: post-arterial capillary, PVC: pre-venular capillary, PCV: post-capillary venule, V: venule, LEC: lymphatic EC, FB: fibroblasts, KC: keratinocytes, LC: Langerhans cells, ILC: innate lymphoid cell.
- ET
Eliza Pei-Suen Tsou, PhD (she/her/hers)
University of Michigan
Ann Arbor, Michigan, United StatesDisclosure information not submitted.
Abstract Poster Presenter(s)
Background/Purpose: Systemic sclerosis (SSc) is characterized by extensive damage of the microvessels in multiple organs. We and others showed that endothelial cells (ECs) isolated from SSc skin biopsies show dysregulated phenotypes including impaired angiogenesis, barrier dysfunction, and spontaneous/constitutive endothelial-to-mesenchymal transition (EndoMT). Notably, these are hallmarks of EC senescence. In this study, we therefore sought to determine the presence and role of EC senescence in SSc skin fibrosis.
Methods: Dermal microvascular ECs were isolated from skin biopsies of age-matched healthy individuals and SSc patients. Cellular senescence was assessed through qPCR, immunofluorescence, β-galactosidase expression and measuring the senescence-associated secretory phenotype (SASP). To investigate EC-specific senescence at the single-cell level, we analyzed skin biopsies from 18 healthy controls and 22 patients with early diffuse SSc. Senescence and extracellular matrix (ECM) scores were constructed using specific gene lists, and receptor-ligand interactions were evaluated. P values under 0.05 were deemed statistically significant.
Results: SSc ECs displayed increased senescence, with higher senescence markers (p16, p53, and β-galactosidase), DNA damage (γH2AX and phospho-ATM), SASP secretion (IL-6, IL-8, PAI-1, MMP1, HGF), and reduced eNOS activity, compared to age-matched healthy ECs. In SSc skin biopsies, senescence marker staining was prominently associated with small blood vessels. Single-cell RNA-seq revealed seven distinct EC clusters; one cluster, expanded in SSc biopsies, showed pronounced EndoMT traits. Most EC clusters exhibited significant senescence scores using the SenMayo gene list, independent of age. CDKN1A, a canonical senescence marker not included in SenMayo, was notably elevated in SSc ECs compared to controls. Cluster-specific senescence scores were highly correlated with ECM scores. Receptor-ligand analysis indicated that EC senescence in SSc was associated with signaling interactions with myofibroblasts and with dendritic cells. Dominant mediators for this cross-talk included the TGF-β, IL-6, and SEMA7 pathways (Figure 1).
Conclusion: By employing a multi-marker, stepwise approach, we identified senescent ECs in SSc skin microvasculature, confirming endothelial senescence as a key element of the disease. On-going studies are investigating the mechanistic role of senescent ECs in SSc-associated immune, vascular and fibrotic pathologies. This unusual EC phenotype may play a crucial pathogenic role in SSc and could serve as a potential target for senotherapy.

